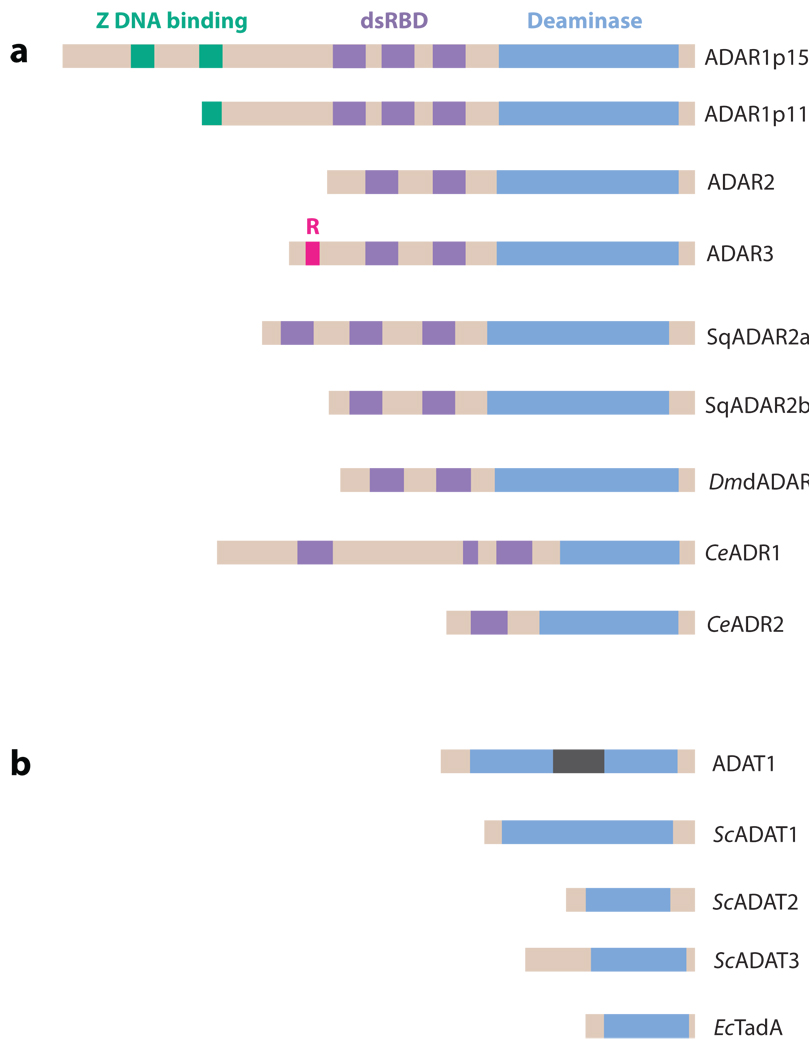
Figure 2.
ADAR and ADAT family members. (a) The ADAR family is shown. Three vertebrate ADAR family members (ADAR1–3) are known. Two ADAR1 translation products (ADAR1p150 and ADAR1p110 isoforms) are known. Vertebrate ADARs, squid SqADAR2a and SqADAR2b (splicing isoforms), Drosophila melanogaster dADAR, and two C. elegans members (CeADR1 and CeADR2) share common functional domains: two to three repeats of the dsRNA-binding domain (dsRBD) and a catalytic deaminase domain. Certain structural features, such as Z-DNA-binding domains and the arginine-rich R domain, are unique to particular ADAR members. (b) The ADAT family is shown. Presented is the only known mammalian ADAT family member (ADAT1), its yeast homolog (ScADAT1), two additional yeast ADAT families (ScADAT2 and ScADAT3), and the only known bacterial ADAT family member (EcTadA). The unique mammalian ADAT1 sequence is located within the deaminase domain (gray bar). ADARs target dsRNA. ADATs target tRNA, even though they lack any known RNA-binding motifs. Yeast ScADAT2 and ScADAT3 form active heterodimers, whereas ADAR1 and ADAR2 form active homodimers.