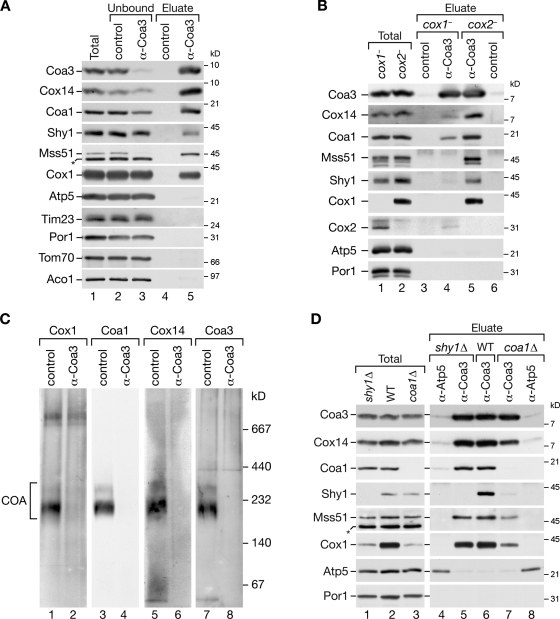
Figure 4.
Formation of COA complexes strictly depends on Cox1; sequestration of Mss51 is independent of Cox2, Shy1, and Coa1. (A) Coimmunoprecipitations of Coa3 from digitonin-solubilized mitochondria. After solubilization, samples were incubated with Coa3-specific or preimmune (control) antisera. Bound material was eluted and analyzed by SDS-PAGE and Western blotting. The amounts of protein loaded in the total and unbound samples correspond to 6% of the eluate. (B) Coimmunoprecipitation experiments as in A were performed in cox1− and cox2− mitochondria. (C) Isolated cox2− mitochondria were solubilized in digitonin and subjected to immunoprecipitation with Coa3 or preimmune (control) antisera. Lysates were depleted from antibody-bound complexes with protein A–Sepharose, and unbound material was separated by BN-PAGE, followed by Western blot analysis. (D) Coimmunoprecipitation experiments from wild-type (WT), shy1Δ, and coa1Δ mitochondria were performed as in A except that anti-Atp5 antiserum was used as a control. (A and D) Asterisks indicate a cross-reactive signal detected by Mss51 antiserum.