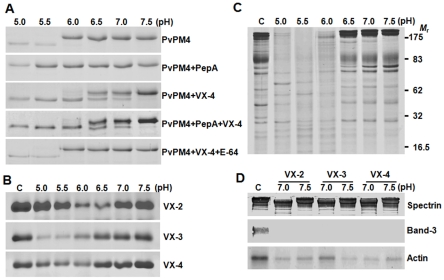
Figure 5. Reactivity of rVX-4 against macromolecular substrates.
(A) Processing of P. vivax plasmepsin 4 (PvPM4) by rVX-4. Recombinant PvPM 4 (20 µg) was incubated with rVX-4 (50 nM) supplemented with 10 mM DTT at different pH values with or without pepstatin A (10 µM) or E-64 (1 µM) for 3 h at 37°C. The reactants were analyzed by 12% SDS-PAGE. (B) Comparison of hemoglobinolytic activity of VX-2, VX-3 and VX-4. Native human hemoglobin was incubated with the respective enzymes in appropriate buffers (pH ranges 5.0–7.5) supplemented with 1 mM GSH for 3 h at 37°C, after which resolved by 10% SDS-PAGE. (C) Hydrolysis of erythrocyte membrane proteins by rVX-4 at different pHs. Fresh erythrocyte ghosts were incubated with rVX-4 in appropriate buffers (pHs 5.0–7.5) for 3 h at 37°C and reaction products were analyzed by 10% SDS-PAGE. Molecular masses in kDa are shown to the right. (D) Western blotting of erythrocyte ghost proteins. The reactions were done at pH 7 and 7.5. The reactants were separated by 10% SDS-PAGE, transferred to a PVDF membrane and probed with specific antibodies against human erythrocyte spectrin (1∶500), band 3 (1∶30000) and actin (1∶1000) followed by horseradish peroxidase conjugated anti-human IgG (1∶1000). The blots were developed with 4C1N. C, control without enzyme.