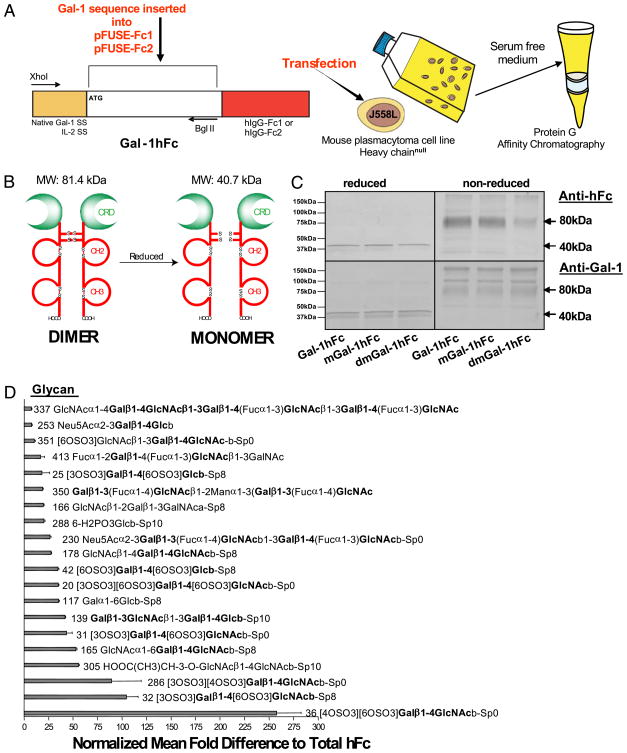
FIGURE 1.
Construction and purification of Gal-1hFc and its mutants. A, Mouse Gal-1 cDNA containing native signal sequence (SS) or IL-2-SS was ligated in-frame into commercially available vector encoding the Fc region of IgG1 (pFUSE-Fc1) or the non-Fc receptor-binding mutant (pFUSE-Fc2), respectively. Purified plasmid DNA was transfected into J558L mouse plasmacytoma cells, drug selected and grown in serum-free medium. Gal-1hFc was purified by protein-G affinity chromatography. B, Schematic representation of Gal-1hFc in its reduced and nonreduced forms. C, Purified Gal-1hFc and its mutants were analyzed by SDS-PAGE and Western blotting with anti-human Fc or anti-mouse Gal-1 mAbs. D, Gal-1hFc or hFc was incubated on a covalent printed glycan array (version 4.0) developed by Core H investigators of the Consortium for Functional Glycomics. Mean fluorescence intensities of Gal-1hFc binding were normalized by dividing Gal-1hFc fluorescence intensities by control hFc-binding intensities and graphed as mean fold difference. The top 20 normalized glycans are listed.