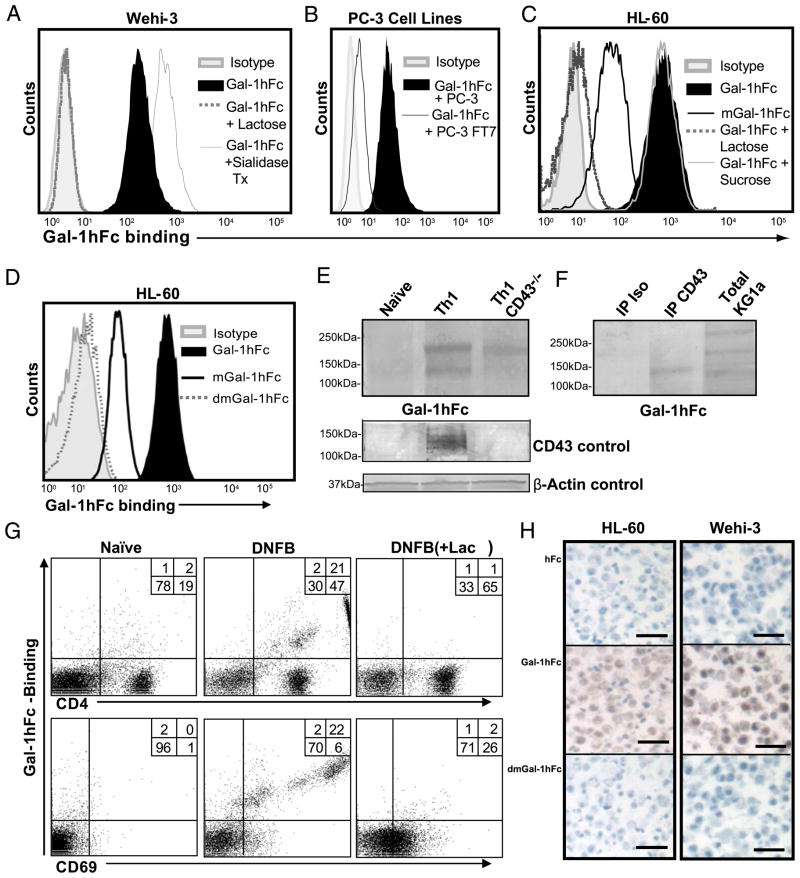
FIGURE 2.
Carbohydrate-binding activity of Gal-1hFc and its mutants to hematopoietic and nonhematopoietic cells by flow cytometry, Western blotting, and immunohistochemistry. A, Gal-1hFc binding to Wehi-3 cells or cells treated with Vibrio cholerae sialidase (0.2 U/ml) for 30 min at 37°C was assessed in the presence or absence of 50 mM lactose. B, Gal-1hFc binding was assayed on PC-3 cells and on PC-3 α1,3 fucosyltransferase 7 (FT7) transfectants (24). C and D, Gal-1hFc, mGal-1hFc, and dmGal-1hFc binding to HL-60 cells was assayed in the presence or absence of 50 mM lactose or sucrose. E, Lysates (30 μg/lane) from naive Th cells or polarized Th1 cells isolated from wt or CD43−/− mice were subjected to reducing 4–20% SDS-PAGE gels, blotted with Gal-1hFc, anti-CD43 mAb (1B11), or anti–β-actin mAb and then with respective AP-secondary Ab. F, KG1a cell lysate (30 μg/lane) and anti-CD43 and isotype control immunoprecipitates from KG1a cells were separated by 4–20% reducing SDS-PAGE gradient gels and then blotted with Gal-1hFc and AP–anti-hFc. G, Lymphocytes from LNs draining DNFB-sensitized or naive skin were analyzed by flow cytometry with Gal-1hFc, and anti-CD4 and -CD69 mAbs. Lactose (+ lac) was added to assay and washing buffers to control for carbohydrate-mediated binding. H, Sections of paraffin-embedded, formalin-fixed HL-60 or Wehi-3 cells were immunostained with 10 μg/ml Gal-1hFc or controls (hFc and dmGal-1hFc). Scale bars, 20 μm. Original magnification ×20. All data are representative of at least three experiments.