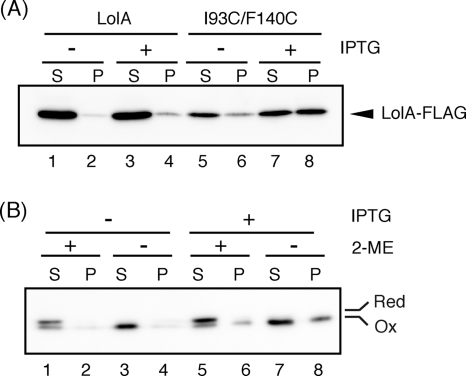
FIG. 5.
Localization of LolA(I93C/F140C). (A) C43(DE3)/pCOLA-CDE cells were transformed with either pSW77 or pSWIF. An overnight culture of the cells was diluted 200-fold with fresh medium containing or not containing 1 mM IPTG, followed by incubation for 2 h. Plasmid-encoded LolA was then induced with 0.2% arabinose for 3 h. Lysates were prepared by sonication from the same amounts of cells and fractionated into supernatant (S) and total membrane (P) fractions by centrifugation at 10,000 × g for 30 min. Equivalent amounts of fractions were analyzed by SDS-PAGE and immunoblotting with anti-FLAG antibodies. (B) Overnight-grown C43(DE3)/pCOLA-CDE/pSWIF cells were incubated in the presence and absence of 1 mM IPTG for 2 h as for panel A. Expression of I93C/F140C was induced with 0.2% arabinose in the presence of 10 mM 2-mercapthoethanol for 3 h. Cells were then collected by centrifugation and washed twice with phosphate-buffered saline to remove the 2-mercapthoethanol. The cells were resuspended in the original volume of fresh medium and then divided into two portions; one portion was treated with 10 mM 2-mercapthoethanol, while the other was not. After a further 30 min of incubation with aeration, cell lysates were prepared from both cultures and fractionated as for panel A. Proteins were analyzed by SDS-PAGE without a reducing agent to separate the oxidized and reduced forms of I93C/F140C, followed by analysis by immunoblotting with anti-FLAG antibodies.