Through the actions of its main biological peptide, angiotensin (Ang) II, the renin-angiotensin system (RAS) has been implicated in atherosclerosis (1, 2). The pro-atherogenic effect of Ang II may involve a variety of pro-inflammatory mechanisms, which are largely independent from its effect on blood pressure (1). Ang II promotes monocyte and endothelial cell activation as well as leukocyte recruitment and adhesion to the atherosclerotic plaque (3). This peptide also increases vascular oxidative stress, an effect which may also contribute to atherogenesis (4).
The formation of Ang II by ACE-dependent and ACE-independent pathways has been viewed as the main determinant of systemic and local Ang II levels. With the discovery of ACE2, an enzyme that degrades Ang II, there has been increased interest in the regulation of Ang II levels through the degradation pathway (5, 6). ACE2 is a homologue of Angiotensin converting enzyme (ACE) that cleaves the octapeptide Ang II into Ang-(1–7) by removing a single amino acid phenylalanine from its C-terminal end (7, 8). Because of its ability to degrade Ang II, ACE2 is viewed as a counter-regulator of Ang II overactivity (8, 9). This property could have therapeutic implications. We suggested that ACE2 could be renoprotective, especially when associated with low levels of ACE (10). Increasing evidence suggests that ACE2 plays a protective role in cardiovascular disease and other pathologies (5, 11).
In this issue, Thomas et al. (12) report their findings of a cross between the atherosclerosis-prone ApoE KO and ACE2 KO mice to study the effects of ACE2 deficiency on the development of atherosclerosis. Genetic ACE2 ablation led to enhanced vascular inflammation and plaque formation in ApoE KO mice. In ApoE replete mice, ACE2 deficiency was also associated with increased expression of inflammatory markers, such as interleukin-6, monocyte chemoattractant protein-1, and vascular cell adhesion molecule-1, suggesting that these differences were indeed related to the deficiency of ACE2 in the vasculature. This important study provides strong evidence for a pro-inflammatory and pro-atherogenic effect associated with deficiency of ACE2 both in vivo and in vitro. What could the mechanism be? In the context of the known pro-inflammatory and pro-atherogenic actions of Ang II, impaired degradation of this peptide owing to ACE2 deficiency could explain their findings. In other words, Ang II accumulation systemically and/or locally within the vasculature could be responsible for the observed increases in vascular inflammation – as seen in the ACE2 KO - and enhanced plaque formation – as seen in the ApoE/ACE2 double KO (12). The issue, however, is complicated by the fact that genetic ACE2 ablation may or may not lead to demonstrable Ang II overactivity as judged by increased levels of circulating or tissue Ang II levels. Marked differences have been reported between three different ACE2-deficient mouse lines in terms of cardiovascular phenotype and basal Ang levels (12–15). The line originally described by Crackower et al. (8), which was used in this study (12), has increased levels of Ang II in kidneys, hearts, and in plasma. By contrast, the other available mouse lines (13, 14) do not have any overt differences in the levels of these peptides. These differences in phenotypes between ACE2-deficient lines cannot be easily explained by differences in the gene disruption methodology or differences in genetic backgrounds (15). ACE2 deficiency in the other two ACE2-deficient lines, however, is not without consequence, rather, the metabolism of Ang II is clearly impaired as demonstrated by increased plasma and kidney levels of Ang II when this peptide is infused. Moreover, this is associated with marked worsening of Ang II-induced hypertension (13, 16).
That treatment with the ACE inhibitor, perindopril, reduced plaque accumulation in ApoE/ACE2 double KO mice, is evidence for the involvement of Ang II in the atherogenic response observed in this model (12). The degree of conferred protection was less than that observed following ACE inhibitor administration to ApoE KO mice. ACE2 activity in the vasculature was decreased following the administration of perindopril (12), which is surprising considering that others have shown increases in ACE2 activity following the administration of either ACE inhibitors or AT1 receptor blockers (18, 19). Nevertheless, the improvement in atherogenesis with the ACE inhibitor implies Ang II dependency and is consistent with the expected impairment in the degradation of this peptide in the face of ACE2 deficiency. It is possible, however, that peptides other than Ang II could also be altered in mice with genetic ACE2 ablation and may contribute to the amplification of vascular inflammation and atherogenesis. For instance, ACE2 removes the C-terminal residue from apelin and des-Arg9-bradykinin, as well as other vasoactive peptides, such as neurotensin and kinetensin (17). The status of these peptides in the ACE2 KO is currently unknown.
Atherosclerotic lesions express matrix metalloproteinases (MMP), which possess proteolytic activity. For instance, MMP-8 has been shown to convert Ang I to Ang II (20). This may provide a mechanism of activation of the RAS locally within the plaque. Along with this concept, Thomas et al. (12) now show that ACE2 KO mice have increased aortic expression of MMP-2 and MMP-9 as well as increased MMP-9 production in macrophages. It is therefore likely that the deficiency of ACE2 leads to excessive Ang II accumulation particularly in the face of MMP-driven Ang II formation. In this way, it would be difficult to escape from Ang II overactivity, which becomes a perpetuating mechanism leading to atherogenesis as a result of both increased formation and decreased degradation of Ang II.
It would have been of interest to know ACE2 expression in the vasculature of ApoE KO mice early on and as the disease progresses. ACE2 is present in atherosclerotic arteries in humans and levels of its activity seem to vary in vessel wall in the course of the lesions progression (21). Although it is still not clear whether its presence reflects a compensatory response to enhanced Ang II activity within the diseased vasculature, increasing ACE2 activity may be therapeutically relevant. A gain of ACE2 activity by adenoviral over-expression exerted beneficial effects in ApoE KO mice fed a lipid-reach diet by reducing atherosclerotic aortic lesions (22). Adenoviral ACE2 overexpression has also been shown to stabilize existing atherosclerotic plaques and attenuate the progression of lesions early on, but not the progression of plaque size at more advanced stages (23).
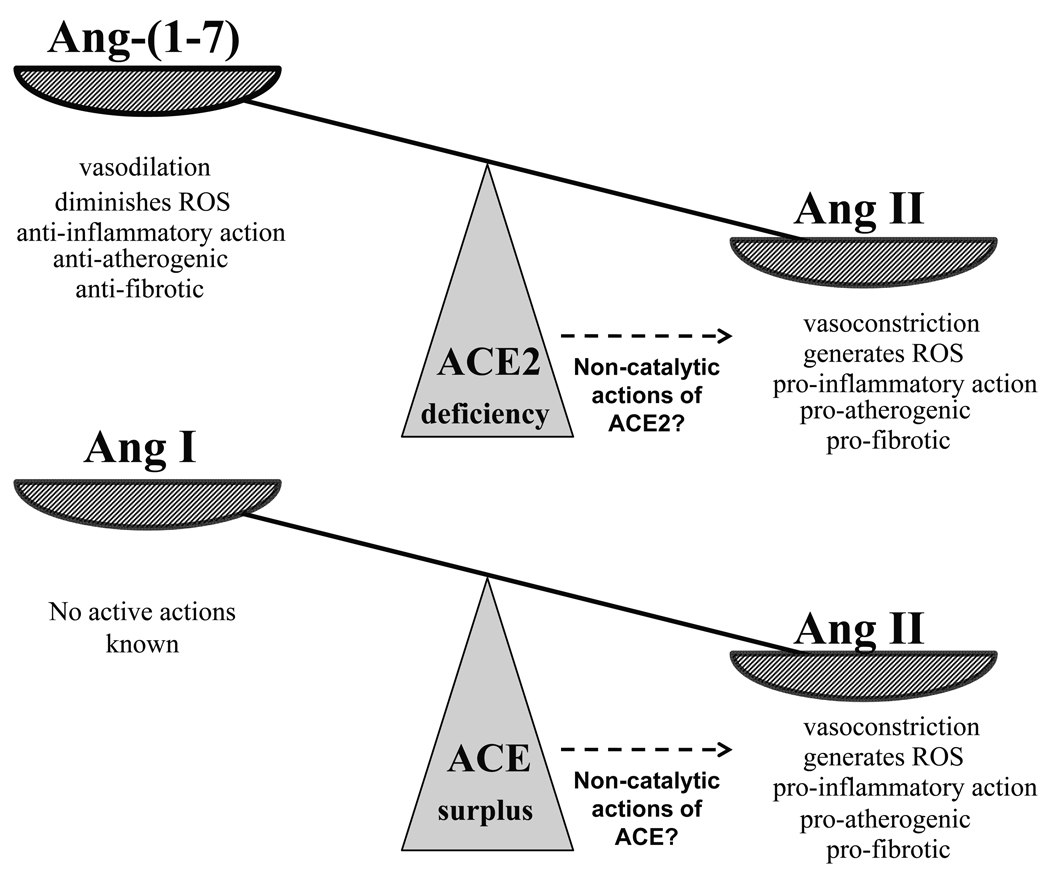
As mentioned above, ACE2 cleavage of Ang II results in the formation of Ang-(1–7), a peptide which is believed to have anti-inflammatory properties (24). The two effects of ACE2: degrading the pro-inflammatory Ang II and promoting formation of anti-inflammatory Ang-(1–7), may be synergistic from the perspective of inflammation (Figure). This raises the question of what causes the observed pro-inflammatory effect of ACE2 deficiency, is it increased Ang II or decreased Ang (1–7), or both? The levels of Ang-(1–7) were not reported by Thomas et al. (12) in the ACE2 KO, but one would expect them to be decreased as a result of diminished ACE2 driven angiotensin II degradation. Angiotensin-(1–7) infusion has been shown to be atheroprotective in ApoE KO mice (24) suggesting a role for this peptide on atherogenesis. At present, however, the relative contribution of Ang II versus Ang-(1–7) regarding atherogenesis is unknown. We have previously shown that angiotensin II rather than angiotensin-(1–7) mediates the antihypertensive effect of recombinant ACE2 protein in the hypertension associated with angiotensin II infusion (16). It will be important to elucidate the relative contribution of these two peptides on atherogenesis, particularly because Ang-(1–7) agonists are now available.
Figure 1.
The impact of ACE2 deficiency on the balance between Angiotensin II and Angiotensin (1–7) is tilted towards Ang II accumulation as a result of impaired Ang II degradation (Upper Panel). ACE surplus also shifts the balance towards Ang II accumulation via increased formation from Ang I (Lower Panel). Some of the known detrimental actions of Ang II are opposed by Ang-(1–7), whereas Ang I has no known biological actions.
There could also be other mechanisms involved in the action of ACE2 in addition to its effect on the degradation of Ang II to Ang 1–7 (Figure). A non-catalytic role for ACE2 has been postulated (9) and cannot be ruled out as being involved in its vascular protective actions. Another question is what happens to ACE activity when ACE2 activity decreases? The administration of MLN-4760, a specific ACE2 inhibitor, has been shown to increase ACE expression in the kidney vasculature and glomeruli (25). It is possible that overactivity of Ang II may originate not only from impaired degradation as a result of ACE2 deficiency but also by enhanced formation resulting from increased ACE activity (Figure). This combination of increased ACE but decreased ACE2 expression has been described in glomeruli from mice models of diabetic kidney disease (26). A state of ACE2 deficiency and ACE excess would potentiate Ang II accumulation by further shifting the balance away from Ang II degradation and in favor of Ang II formation (Figure).
In the future, it will be important to evaluate the anti-atherogenetic and anti-inflammatory response to interventions aimed at amplifying ACE2 activity directly, such as the administration of recombinant ACE2 protein. Moreover, a combination of approaches, blocking Ang II formation and increasing Ang II degradation via ACE2 administration, should have an additive effect on reducing plaque formation. Since in clinical studies the effect of blockade of RAS has been relatively modest in terms of plaque reduction (27), it would seem that a more effective approach may involve amplification of ACE2 activity alone or perhaps in combination with RAS blockade to protect the vascular ring.
Acknowledgments
Sources of Funding: Dr. Daniel Batlle has active grant support from NIDDK (1R01DK080089-01A2) and JDRF exploring ACE2 amplification by various approaches as a therapeutic strategy for animal models of diabetic kidney disease.
Non-Standard Abbreviations and Acronyms
- ACE
angiotensin-converting enzyme
- ACE2
angiotensin-converting enzyme 2
- Ang-(1–7)
angiotensin-(1–7)
- Ang II
angiotensin II
- ApoE
apolipoprotein E
- KO
knockout
- Perindopril
angiotensin-converting enzyme inhibitor
- MLN-4760
angiotensin-converting enzyme 2 inhibitor
- MMP
matrix metalloproteinase
- RAS
renin-angiotensin system
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Dr. Batlle has a pending patent application entitled ‘Methods for Achieving a Protective ACE2 Expression Level to Treat Kidney Disease and Hypertension.’
References
- 1.Daugherty A, Manning MW, Cassis LA. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J. Clin. Invest. 2000;105(11):1605–1612. doi: 10.1172/JCI7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Libby P. Inflammation in atherosclerosis. Nature. 2002;420(6917):868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 3.Ekholm M, Kahan T, Jörneskog G, Bröijersen A, Wallén NH. Angiotensin II infusion in man is proinflammatory but has no short-term effects on thrombin generation in vivo. Thromb. Res. 2009;124(1):110–115. doi: 10.1016/j.thromres.2008.12.040. [DOI] [PubMed] [Google Scholar]
- 4.Nickenig G, Harrison DG. The AT1-Type Angiotensin Receptor in Oxidative Stress and Atherogenesis: Part I: Oxidative Stress and Atherogenesis. Circulation. 2002;105(3):393–396. doi: 10.1161/hc0302.102618. [DOI] [PubMed] [Google Scholar]
- 5.Batlle D, Soler MJ, Wysocki J. New aspects of the renin-angiotensin system: angiotensin-converting enzyme 2 - a potential target for treatment of hypertension and diabetic nephropathy. Curr. Opin. Nephrol. Hypertens. 2008;17(3):250–257. doi: 10.1097/MNH.0b013e3282f945c2. [DOI] [PubMed] [Google Scholar]
- 6.Ferreira AJ, Santos RA, Bradford CN, Mecca AP, Sumners C, Katovich MJ, Raizada MK. Therapeutic Implications of the Vasoprotective Axis of the Renin-Angiotensin System in Cardiovascular Diseases. Hypertension. 2010;55(2):207–213. doi: 10.1161/HYPERTENSIONAHA.109.140145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, Donovan M, Woolf B, Robison K, Jeyaseelan R, Breitbart RE, Acton S. A Novel Angiotensin-Converting Enzyme-Related Carboxypeptidase (ACE2) Converts Angiotensin I to Angiotensin 1–9. Circ Res. 2000;87(5):e1–e9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 8.Crackower MA, Sarao R, Oudit GY, Yagil C, Kozieradzki I, Scanga SE, Oliveira-dos-Santos AJ, da Costa J, Zhang L, Pei Y, Scholey J, Ferrario CM, Manoukian AS, Chappell MC, Backx PH, Yagil Y, Penninger JM. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 2002;417(6891):822–828. doi: 10.1038/nature00786. [DOI] [PubMed] [Google Scholar]
- 9.Lambert D, Clarke N, Turner A. Not just angiotensinases: new roles for the angiotensin-converting enzymes. Cellular and Molecular Life Sciences. 2010;67(1):89–98. doi: 10.1007/s00018-009-0152-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ye M, Wysocki J, Naaz P, Salabat MR, LaPointe MS, Batlle D. Increased ACE 2 and Decreased ACE Protein in Renal Tubules From Diabetic Mice: A Renoprotective Combination? Hypertension. 2004;43(5):1120–1125. doi: 10.1161/01.HYP.0000126192.27644.76. [DOI] [PubMed] [Google Scholar]
- 11.Danilczyk U, Penninger JM. Angiotensin-Converting Enzyme II in the Heart and the Kidney. Circ Res. 2006;98(4):463–471. doi: 10.1161/01.RES.0000205761.22353.5f. [DOI] [PubMed] [Google Scholar]
- 12.Thomas MC, Pickering RJ, Tsorotes D, Koitka A, Sheehy K, Bernardi S, Toffoli B, Nguyen-Huu T, Head G, Fu Y, Chin-Dusting J, Cooper ME, Tikellis C. Genetic Ace2 Deficiency Accentuates Vascular Inflammation and Atherosclerosis in the ApoE Knockout Mouse. Circ Res. 2010;107 doi: 10.1161/CIRCRESAHA.110.219279. XXX-XXX. [DOI] [PubMed] [Google Scholar]
- 13.Gurley SB, Allred A, Le TH, Griffiths R, Mao L, Philip N, Haystead TA, Donoghue M, Breitbart RE, Acton SL, Rockman HA, Coffman TM. Altered blood pressure responses and normal cardiac phenotype in ACE2-null mice. J. Clin. Invest. 2006;116(8):2218–2225. doi: 10.1172/JCI16980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamamoto K, Ohishi M, Katsuya T, Ito N, Ikushima M, Kaibe M, Tatara Y, Shiota A, Sugano S, Takeda S, Rakugi H, Ogihara T. Deletion of Angiotensin-Converting Enzyme 2 Accelerates Pressure Overload-Induced Cardiac Dysfunction by Increasing Local Angiotensin II. Hypertension. 2006;47(4):718–726. doi: 10.1161/01.HYP.0000205833.89478.5b. [DOI] [PubMed] [Google Scholar]
- 15.Gurley SB, Coffman TM. Angiotensin-converting enzyme 2 gene targeting studies in mice: mixed messages. Exp. Physiol. 2008;93(5):538–542. doi: 10.1113/expphysiol.2007.040014. [DOI] [PubMed] [Google Scholar]
- 16.Wysocki J, Ye M, Rodriguez E, González-Pacheco FR, Barrios C, Evora K, Schuster M, Loibner H, Brosnihan KB, Ferrario CM, Penninger JM, Batlle D. Targeting the degradation of angiotensin II with recombinant angiotensin-converting enzyme 2: prevention of angiotensin II-dependent hypertension. Hypertension. 2010;55(1):90–98. doi: 10.1161/HYPERTENSIONAHA.109.138420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalea AZ, Batlle D. Apelin and ACE2 in cardiovascular disease. Curr Opin Investig Drugs. 2010;11(3):273–282. [PubMed] [Google Scholar]
- 18.Soler MJ, Ye M, Wysocki J, William J, Lloveras J, Batlle D. Localization of ACE2 in the renal vasculature: amplification by angiotensin II type 1 receptor blockade using telmisartan. Am. J. Physiol. Renal Physiol. 2009;296(2):F398–F405. doi: 10.1152/ajprenal.90488.2008. [DOI] [PubMed] [Google Scholar]
- 19.Ferrario CM, Jessup J, Chappell MC, Averill DB, Brosnihan KB, Tallant EA, Diz DI, Gallagher PE. Effect of Angiotensin-Converting Enzyme Inhibition and Angiotensin II Receptor Blockers on Cardiac Angiotensin-Converting Enzyme 2. Circulation. 2005;111(20):2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- 20.Laxton RC, Hu Y, Duchene J, Zhang F, Zhang Z, Leung K, Xiao Q, Scotland RS, Hodgkinson CP, Smith K, Willeit J, Lopez-Otin C, Simpson IA, Kiechl S, Ahluwalia A, Xu Q, Ye S. A Role of Matrix Metalloproteinase-8 in Atherosclerosis. Circ Res. 2009;105(9):921–929. doi: 10.1161/CIRCRESAHA.109.200279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sluimer J, Gasc J, Hamming I, van Goor H, Michaud A, van den Akker L, Jütten B, Cleutjens J, Bijnens A, Corvol P, Daemen M, Heeneman S. Angiotensin-converting enzyme 2 (ACE2) expression and activity in human carotid atherosclerotic lesions. J. Pathol. 2008;215(3):273–279. doi: 10.1002/path.2357. [DOI] [PubMed] [Google Scholar]
- 22.Lovren F, Pan Y, Quan A, Teoh H, Wang G, Shukla PC, Levitt KS, Oudit GY, Al-Omran M, Stewart DJ, Slutsky AS, Peterson MD, Backx PH, Penninger JM, Verma S. Angiotensin converting enzyme-2 confers endothelial protection and attenuates atherosclerosis. Am J Physiol Heart Circ Physiol. 2008;295(4):H1377–H1384. doi: 10.1152/ajpheart.00331.2008. [DOI] [PubMed] [Google Scholar]
- 23.Dong B, Zhang C, Feng JB, Zhao YX, Li SY, Yang YP, Dong QL, Deng BP, Zhu L, Yu QT, Liu CX, Liu B, Pan CM, Song HD, Zhang MX, Zhang Y. Overexpression of ACE2 Enhances Plaque Stability in a Rabbit Model of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28(7):1270–1276. doi: 10.1161/ATVBAHA.108.164715. [DOI] [PubMed] [Google Scholar]
- 24.Tesanovic S, Vinh A, Gaspari TA, Casley D, Widdop RE. Vasoprotective and Atheroprotective Effects of Angiotensin (1–7) in Apolipoprotein E-Deficient Mice. Arterioscler Thromb Vasc Biol. 2010;30(8):1606–1613. doi: 10.1161/ATVBAHA.110.204453. [DOI] [PubMed] [Google Scholar]
- 25.Soler MJ, Wysocki J, Ye M, Lloveras J, Kanwar Y, Batlle D. ACE2 inhibition worsens glomerular injury in association with increased ACE expression in streptozotocin-induced diabetic mice. Kidney Int. 2007;72(5):614–623. doi: 10.1038/sj.ki.5002373. [DOI] [PubMed] [Google Scholar]
- 26.Ye M, Wysocki J, William J, Soler MJ, Cokic I, Batlle D. Glomerular localization and expression of Angiotensin-converting enzyme 2 and Angiotensin-converting enzyme: implications for albuminuria in diabetes. J. Am. Soc. Nephrol. 2006;17(11):3067–3075. doi: 10.1681/ASN.2006050423. [DOI] [PubMed] [Google Scholar]
- 27.Stumpe KO, Agabiti-Rosei E, Zielinski T, Schremmer D, Scholze J, Laeis P, Schwandt P, Ludwig M. Carotid intima-media thickness and plaque volume changes following 2-year angiotensin II-receptor blockade. The Multicentre Olmesartan atherosclerosis Regression Evaluation (MORE) study. Ther Adv Cardiovasc Dis. 2007;1(2):97–106. doi: 10.1177/1753944707085982. [DOI] [PubMed] [Google Scholar]