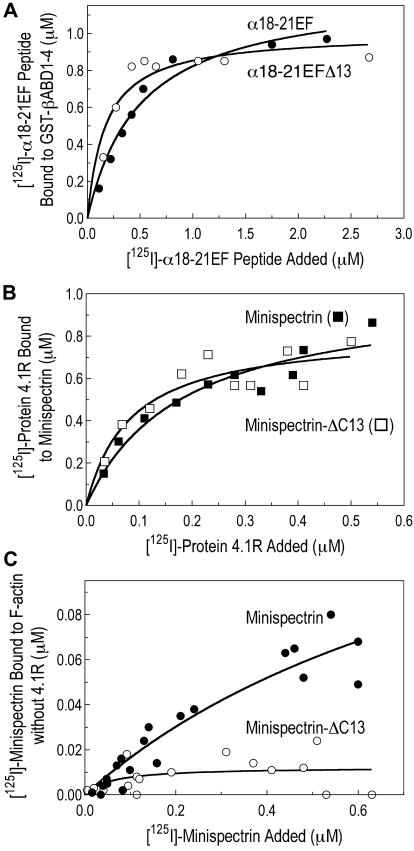
Figure 5.
Heterodimer assembly and protein 4.1R binding. (A) Assembly of the minispectrin heterodimer. GST-βABD1-4 (0.87μM) was mixed with increasing amounts of [125I]-α18-21EF or α18-21EFΔ13 (0.14-2.12μM), and the complex was retrieved on GSH beads as described in “GST pull-down experiments” (B) Protein 4.1R binding to minispectrin. Increasing amounts of [125I]-protein 4.1 (0.18-1.36μM) were added to GST-minispectrin or GST-minispectrin-Δ13 (each 0.54μM). In each experiment, binding of the [125I]-ligand to GSH beads alone was subtracted from the values with added spectrin. (C) Minispectrin binding to F-actin in the absence of protein 4.1R. Increasing amounts of [125I]-minispectrin or [125I]-minispectrin-Δ13 were added to F-actin (2.38μM as G-actin), and the bound minispectrin was measured after pelleting the F-actin. Results from 3 independent experiments are combined. Note that the diminished ability of the mutant minispectrin to form the ternary complex is not a failure to assemble the minispectrin molecule or to bind protein 4.1R, but a failure to bind to F-actin.