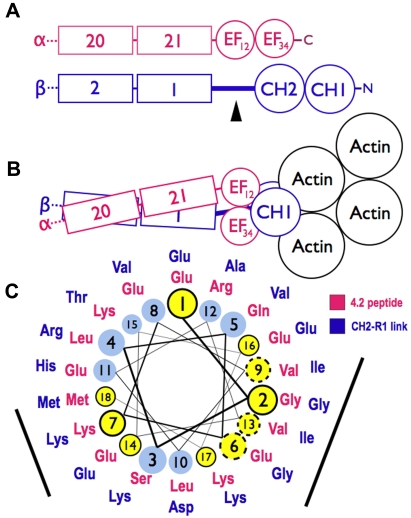
Figure 7.
Models of spectrin-actin interactions based on the structure of α-actinin. (A) Hypothetical models, drawn roughly proportional to size, of α-spectrin (red) and β-spectrin (blue) chains near the tail end of the molecule. EF34 extends to the C-terminus of α-spectrin and is partially deleted in the sph mutation. The black arrowhead marks the segment between the CH2 domain and the first β-spectrin repeat (CH2-R1). (B) Model of the interaction of α- and β-spectrin deduced from the structure of skeletal muscle α-actinin.25 The view is from above, looking down on the membrane skeleton, with the actin filament lying parallel to the lipid bilayer. Note that in this structure the EF domains are turned 90 degrees relative to the CH domains and do not contact the actin filament. They lie near the linker separating the CH domains and the long CH2-R1 linker. The orientation of the CH domains varies from an open to closed conformation (here shown closed) in different actin-binding proteins, but the EF domain is always in contact with the CH domains and not the actin. (C) Helical wheel illustrating the similarity between the spectrin-binding peptide in protein 4.2 (red sequence),33 which binds to the EF domain of α-spectrin,19 and the CH2-R1 linker region in β-spectrin (blue sequence). Both peptides are predicted to form an α-helix. Solid black circles and dashed circles around yellow amino acid positions represent identical and conserved residues, respectively. The black bars represent 2 faces of the putative helices that are notably similar. We postulate that the CH2-R1 linker region of β-spectrin also binds to the EF hands, probably in the groove of EF34, and that this interaction regulates the protein 4.1R and/or actin binding of the adjacent CH domains.