Abstract
The effect of alveolar oxygen tension on lung lipid peroxidation during lung ischemia was evaluated by using isolated rat lungs perfused with synthetic medium. After a 5-min equilibration period, global ischemia was produced by discontinuing perfusion while ventilation continued with gas mixtures containing 5% CO2 and a fixed oxygen concentration between 0 and 95%. Lipid peroxidation was assessed by measurement of tissue thiobarbituric acid-reactive products and conjugated dienes. Control studies (no ischemia) showed no change in parameters of lipid peroxidation during 1 h of perfusion and ventilation with 20% or 95% O2. With 60 min of ischemia, there was increased lipid peroxidation which varied with oxygen content of the ventilating gas and was markedly inhibited by ventilation with N2. Perfusion with 5-, 8-, 11-, 14-eicosatetraynoic acid indicated that generation of eicosanoids during ischemia accounted for approximately 40-50% of lung lipid peroxide production. Changes of CO2 content of the ventilating gas (to alter tissue pH) or of perfusate glucose concentration had no effect on lipid peroxidation during ischemia, but perfusion at 8% of the normal flow rate prevented lipid peroxidation. Lung dry/wet weight measured after 3 min of reperfusion showed good correlation between lung fluid accumulation and lipid peroxidation. These results indicate that reperfusion is not necessary for lipid peroxidation with ischemic insult of the lung and provide evidence that elevated PO2 during ischemia accelerates the rate of tissue injury.
Full text
PDF
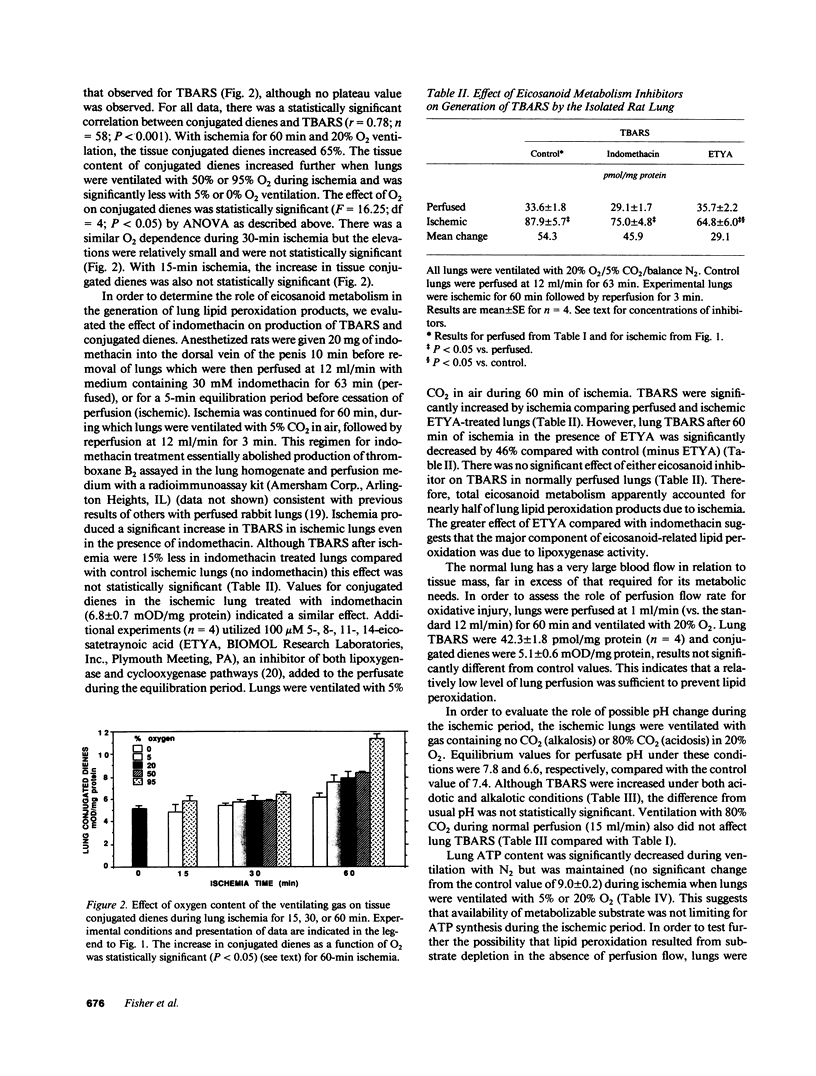
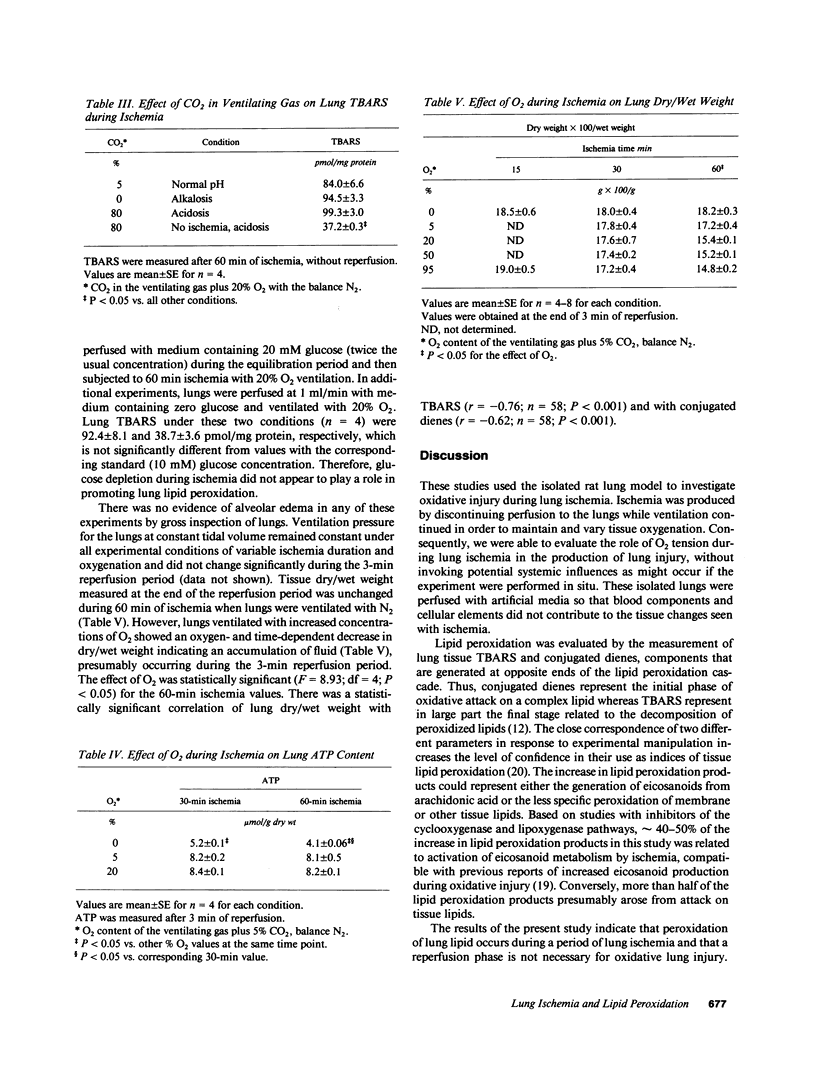


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Barie P. S., Hakim T. S., Malik A. B. Effect of pulmonary artery occlusion and reperfusion on extravascular fluid accumulation. J Appl Physiol Respir Environ Exerc Physiol. 1981 Jan;50(1):102–106. doi: 10.1152/jappl.1981.50.1.102. [DOI] [PubMed] [Google Scholar]
- Bassett D. J., Fisher A. B. Metabolic response to carbon monoxide by isolated rat lungs. Am J Physiol. 1976 Mar;230(3):658–663. doi: 10.1152/ajplegacy.1976.230.3.658. [DOI] [PubMed] [Google Scholar]
- Bishop M. J., Boatman E. S., Ivey T. D., Jordan J. P., Cheney F. W. Reperfusion of ischemic dog lung results in fever, leukopenia, and lung edema. Am Rev Respir Dis. 1986 Oct;134(4):752–756. doi: 10.1164/arrd.1986.134.4.752. [DOI] [PubMed] [Google Scholar]
- Bishop M. J., Chi E. Y., Cheney F. W., Jr Lung reperfusion in dogs causes bilateral lung injury. J Appl Physiol (1985) 1987 Sep;63(3):942–950. doi: 10.1152/jappl.1987.63.3.942. [DOI] [PubMed] [Google Scholar]
- Block E. R., Fisher A. B. Depression of serotonin clearance by rat lungs during oxygen exposure. J Appl Physiol Respir Environ Exerc Physiol. 1977 Jan;42(1):33–38. doi: 10.1152/jappl.1977.42.1.33. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Buege J. A., Aust S. D. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- Clark J. M., Lambertsen C. J. Pulmonary oxygen toxicity: a review. Pharmacol Rev. 1971 Jun;23(2):37–133. [PubMed] [Google Scholar]
- Cross C. E., Halliwell B., Borish E. T., Pryor W. A., Ames B. N., Saul R. L., McCord J. M., Harman D. Oxygen radicals and human disease. Ann Intern Med. 1987 Oct;107(4):526–545. doi: 10.7326/0003-4819-107-4-526. [DOI] [PubMed] [Google Scholar]
- Deeb G. M., Grum C. M., Lynch M. J., Guynn T. P., Gallagher K. P., Ljungman A. G., Bolling S. F., Morganroth M. L. Neutrophils are not necessary for induction of ischemia-reperfusion lung injury. J Appl Physiol (1985) 1990 Jan;68(1):374–381. doi: 10.1152/jappl.1990.68.1.374. [DOI] [PubMed] [Google Scholar]
- Farrukh I. S., Michael J. R., Peters S. P., Sciuto A. M., Adkinson N. F., Jr, Freeland H. S., Paky A., Spannhake E. W., Summer W. R., Gurtner G. H. The role of cyclooxygenase and lipoxygenase mediators in oxidant-induced lung injury. Am Rev Respir Dis. 1988 Jun;137(6):1343–1349. doi: 10.1164/ajrccm/137.6.1343. [DOI] [PubMed] [Google Scholar]
- Fisher A. B., Dodia C., Linask J. Perfusate composition and edema formation in isolated rat lungs. Exp Lung Res. 1980 Mar;1(1):13–21. doi: 10.3109/01902148009057509. [DOI] [PubMed] [Google Scholar]
- Fisher A. B., Furia L., Berman H. Metabolism of rat granular pneumocytes isolated in primary culture. J Appl Physiol Respir Environ Exerc Physiol. 1980 Oct;49(4):743–750. doi: 10.1152/jappl.1980.49.4.743. [DOI] [PubMed] [Google Scholar]
- Fisher A. B., Hyde R. W., Reif J. S. Insensitivity of the alveolar septum to local hypoxia. Am J Physiol. 1972 Oct;223(4):770–776. doi: 10.1152/ajplegacy.1972.223.4.770. [DOI] [PubMed] [Google Scholar]
- Gacad G., Massaro D. Hyperoxia: influence on lung mechanics and protein synthesis. J Clin Invest. 1973 Mar;52(3):559–565. doi: 10.1172/JCI107216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger D. N., McCord J. M., Parks D. A., Hollwarth M. E. Xanthine oxidase inhibitors attenuate ischemia-induced vascular permeability changes in the cat intestine. Gastroenterology. 1986 Jan;90(1):80–84. doi: 10.1016/0016-5085(86)90078-8. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Oxidants and human disease: some new concepts. FASEB J. 1987 Nov;1(5):358–364. [PubMed] [Google Scholar]
- Kennedy T. P., Rao N. V., Hopkins C., Pennington L., Tolley E., Hoidal J. R. Role of reactive oxygen species in reperfusion injury of the rabbit lung. J Clin Invest. 1989 Apr;83(4):1326–1335. doi: 10.1172/JCI114019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama I., Toung T. J., Rogers M. C., Gurtner G. H., Traystman R. J. O2 radicals mediate reperfusion lung injury in ischemic O2-ventilated canine pulmonary lobe. J Appl Physiol (1985) 1987 Jul;63(1):111–115. doi: 10.1152/jappl.1987.63.1.111. [DOI] [PubMed] [Google Scholar]
- McCord J. M. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985 Jan 17;312(3):159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- Mickel H. S., Vaishnav Y. N., Kempski O., von Lubitz D., Weiss J. F., Feuerstein G. Breathing 100% oxygen after global brain ischemia in Mongolian Gerbils results in increased lipid peroxidation and increased mortality. Stroke. 1987 Mar-Apr;18(2):426–430. doi: 10.1161/01.str.18.2.426. [DOI] [PubMed] [Google Scholar]
- Petruska J. M., Wong S. H., Sunderman F. W., Jr, Mossman B. T. Detection of lipid peroxidation in lung and in bronchoalveolar lavage cells and fluid. Free Radic Biol Med. 1990;9(1):51–58. doi: 10.1016/0891-5849(90)90049-o. [DOI] [PubMed] [Google Scholar]
- Salaris S. C., Babbs C. F. Effect of oxygen concentration on the formation of malondialdehyde-like material in a model of tissue ischemia and reoxygenation. Free Radic Biol Med. 1989;7(6):603–609. doi: 10.1016/0891-5849(89)90141-x. [DOI] [PubMed] [Google Scholar]
- Smith C. V., Anderson R. E. Methods for determination of lipid peroxidation in biological samples. Free Radic Biol Med. 1987;3(5):341–344. doi: 10.1016/s0891-5849(87)80044-8. [DOI] [PubMed] [Google Scholar]
- Thom S. R. Antagonism of carbon monoxide-mediated brain lipid peroxidation by hyperbaric oxygen. Toxicol Appl Pharmacol. 1990 Sep 1;105(2):340–344. doi: 10.1016/0041-008x(90)90195-z. [DOI] [PubMed] [Google Scholar]
- Udassin R., Ariel I., Haskel Y., Kitrossky N., Chevion M. Salicylate as an in vivo free radical trap: studies on ischemic insult to the rat intestine. Free Radic Biol Med. 1991;10(1):1–6. doi: 10.1016/0891-5849(91)90014-t. [DOI] [PubMed] [Google Scholar]