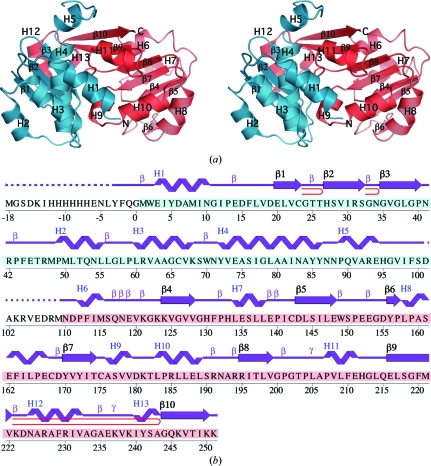
Figure 1.
Crystal structure of Dhaf4260 from D. hafniense. (a) Stereo ribbon diagram of the Dhaf4260 monomer. The N-terminal domain is colored cyan and the C-terminal domain is colored salmon. Helices H1–H13 and β-strands β1–β10 are indicated. (b) Diagram showing the secondary-structure elements of Dhaf4260 superimposed on its primary sequence. The designation of secondary-structure elements is in accord with PDBsum (http://www.ebi.ac.uk/pdbsum). For Dhaf4260, helices are labeled sequentially (H1, H2, H3 etc.) with α-helices H1–H6, H8–H10 and H12 and 310-helices H7, H11 and H13, β-strands are numbered sequentially (strands β1–β3 form the first sheet and strands β4–β10 form the second sheet), β-turns are labeled β, γ-turns are labeled γ and β-hairpins are indicated as red loops. The unmodeled sequence, which is disordered in the electron-density map, is indicated by a dashed line. Residues from the N-terminal domain are highlighted in cyan and residues from the C-terminal domain are in salmon.