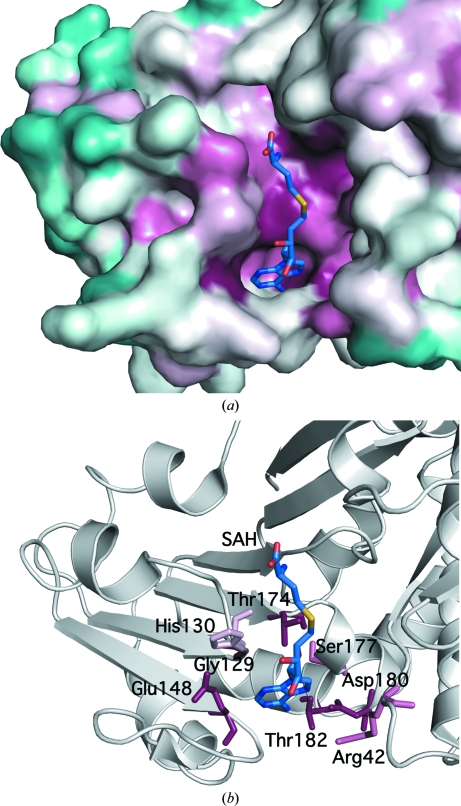
Figure 3.
The interdomain pocket forms a unique catalytic site. (a) Surface representation of the Dhaf4260 domain interface colored by sequence conservation according to ConSurf (Landau et al., 2005 ▶). High conservation among DUF364 homologs is indicated in maroon and low conservation is indicated in turquoise. A docked S-adenosyl-l-homocysteine (SAH) molecule is shown in ball-and-stick representation. Docking was based on its superposition with MT0146 (PDB code 1l3i; Keller et al., 2002 ▶). (b) Ribbon representation of Dhaf4260 in the same orientation as in (a). Highly conserved Dhaf4260 residues are shown in ball-and-stick representation and are labeled.