Table 1. Summary of crystal parameters, data collection and refinement statistics for PA1994 (PDB code 2h1t).
Values in parentheses are for the highest resolution shell.
| λ1 MADSe | λ2 MADSe | λ3 MADSe | |
|---|---|---|---|
| Space group | C2 | ||
| Unit-cell parameters (Å, °) | a = 130.03, b = 41.90, c = 78.65, β = 91.2 | ||
| Data collection | |||
| Wavelength (Å) | 0.9793 | 0.9789 | 0.9116 |
| Resolution range (Å) | 28.3–1.80 (1.85–1.80) | 28.3–1.91 (1.96–1.91) | 28.3–1.80 (1.85–1.80) |
| No. of observations | 136388 | 121791 | 146173 |
| No. of unique reflections | 38719 | 33103 | 39473 |
| Completeness (%) | 98.0 (83.9) | 99.6 (97.2) | 99.7 (98.3) |
| Mean I/σ(I) | 9.9 (1.9) | 10.6 (3.4) | 10.3 (2.6) |
| Rmerge on I† (%) | 9.9 (51.4) | 10.5 (35.1) | 9.9 (51.7) |
| Model and refinement statistics | |||
| Resolution range (Å) | 28.3–1.80 | ||
| No. of reflections (total) | 35699‡ | ||
| No. of reflections (test) | 1772 | ||
| Completeness (%) | 90.2 | ||
| Data set used in refinement | λ1 MADSe | ||
| Cutoff criterion | |F| > 0 | ||
| Rcryst§ | 0.170 | ||
| Rfree¶ | 0.213 | ||
| Stereochemical parameters | |||
| Restraints (r.m.s.d. observed) | |||
| Bond angles (°) | 1.58 | ||
| Bond lengths (Å) | 0.015 | ||
| Average isotropic B value (Å2) | 20.5†† | ||
| ESU‡‡ based on Rfree (Å) | 0.13 | ||
| Protein residues/atoms | 370/3051 | ||
| Waters/other solvent molecules | 367/11 | ||
R
merge = 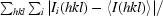
 .
.
Owing to ice rings, a total of 3016 reflections were omitted from the resolution ranges 1.91–1.93, 2.02–2.04 and 2.23–2.27 Å. Typically, a few reflections were also excluded owing to negative intensities and rounding errors in the resolution limits and unit-cell parameters.
R
cryst = 
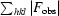 , where F
calc and F
obs are the calculated and observed structure-factor amplitudes, respectively
, where F
calc and F
obs are the calculated and observed structure-factor amplitudes, respectively
R free is the same as R cryst but for 5.0% of the total reflections chosen at random and omitted from refinement.
This represents the total B including both the TLS and residual B components.
