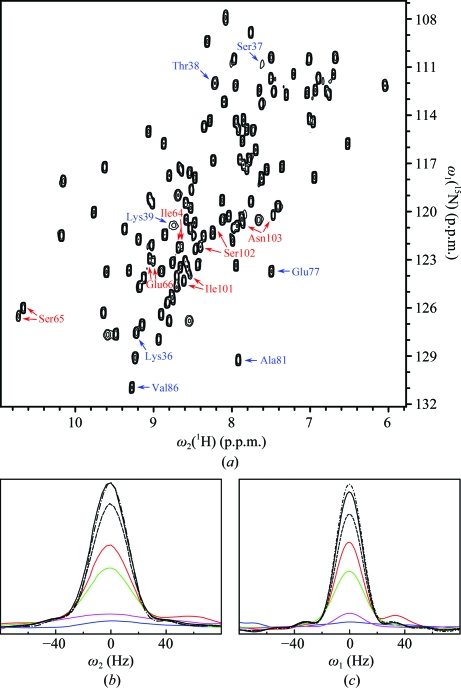
Figure 7.
NMR evidence for slow conformational exchange between several locally different conformations formed by the polypeptide segment 36–39 (see also Fig. 6 ▶ c) and for the coexistence of two conformational species with distinct NMR signals for the polypeptide segments 64–66 and 101–104 (see also Fig. 8 ▶). (a) 2D [15N,1H]-HSQC spectrum of a 0.9 mM solution of NP_247299.1 recorded at 800 MHz and 313 K. The cross-peaks of the residues involved in the aforementioned conformational polymorphisms are identified with the following color code: blue, residues 36–39 and, for reference, Glu77, Ala81 and Val86 [see (b) and (c) below]; red, residues 64–66 and 101–103, which all show two signals (see Fig. 8 ▶). (b, c) NMR line-shape analysis reveals slow conformational exchange between the different conformations of the polypeptide segment 36–39 shown in Fig. 6 ▶(c). (b) and (c) show cross-sections along ω2(1H) and ω1(15N), respectively, illustrating pronounced line broadening of the cross-peaks belonging to Lys36 (red), Ser37 (blue), Thr38 (green) and Lys39 (magenta) when compared with the reference peaks of Glu77 (black), Ala81 (black dashed line) and Val86 (black dashed/dotted line).