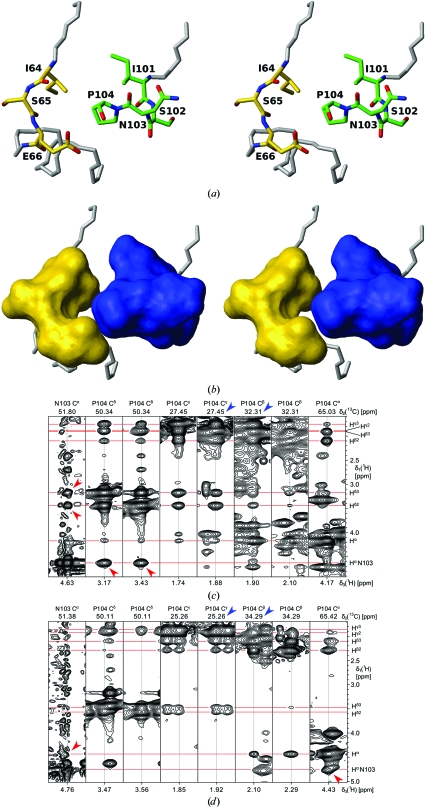
Figure 8.
Cis–trans isomerization of Pro104. (a) Stick representation of all heavy atoms of the polypeptide segments 64–66 and 101–104 for which peak doubling was observed in the 2D [15N,1H]-HSQC spectrum of Fig. 7 ▶(a). The structure containing trans Pro104 is shown. C atoms are colored yellow (residues 64–66) or green (residues 101–104), N atoms are colored blue and O atoms are colored red. Stretches of polypeptide backbone outside of these segments are colored gray. (b) Surface view of the two segments in (a) colored in yellow and blue, respectively. (c, d) Identification of the cis and trans conformations in NP_247299.1 based on 13C chemical shifts and 1H–1H NOEs. (c) and (d) show strips from a 3D 13C(ali)-resolved [1H,1H]-NOESY spectrum representing the signals of the trans and cis forms of the Asn103–Pro104 peptide bond, respectively. (c) The trans form of Pro104 is manifested by d αδ NP NOE connectivities (red arrows) and by the typical 13Cβ and 13Cγ chemical shift pattern (blue arrows). (d) The cis form of Pro104 is manifested by d αα NP NOE peaks (red arrows) and the large difference of about 9 p.p.m. between the 13Cβ and 13Cγ chemical shifts (blue arrows).