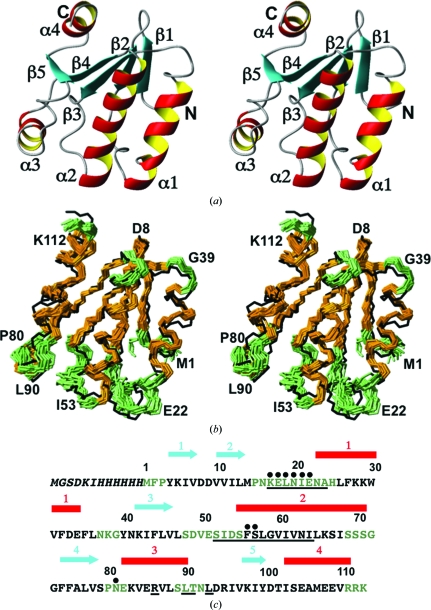
Figure 1.
NMR structure of TM1081 and comparison with the crystal structure. (a) Stereo ribbon diagram of the NMR conformer closest to the mean coordinates of the bundle of conformers in (b). Color code: β-strands, cyan; helices, red/yellow; nonregular secondary structure, gray. The individual regular secondary structures are labeled and the N- and C-termini are indicated. (b) Stereoview of a superposition for best fit of the polypeptide-backbone heavy atoms of residues 3–110 of the crystal structure (black line) with the bundle of 20 conformers that represent the NMR structure. Color code used for the NMR structure: brown, residues with  ≤ 0.50 Å, which is the mean value of the global per-residue displacements in the entire protein; green, residues with
≤ 0.50 Å, which is the mean value of the global per-residue displacements in the entire protein; green, residues with  > 0.50 Å. (c) Amino-acid sequence of the construct used for the NMR structure determination. Black letters represent residues with
> 0.50 Å. (c) Amino-acid sequence of the construct used for the NMR structure determination. Black letters represent residues with  ≤ 0.50 Å and green letters those with
≤ 0.50 Å and green letters those with  > 0.50 Å. The N-terminal segment indicated in italics originates from the expression and purification tag; it was present during the NMR measurements, but is not part of the TM1081 protein and is not shown in (a) and (b). Underlined residues were identified as being conserved in anti-σ factor antagonists (see text) in a sequence-homology search by BLAST and were subsequently confirmed using the ConSurf server (Ashkenazy et al., 2010 ▶). Black dots indicate residues for which no backbone amide resonances were observed in the 2D [15N,1H]-HSQC spectrum. Above the sequence, cyan arrows indicate the positions of the β-strands and red bars those of the α-helices in the NMR structure.
> 0.50 Å. The N-terminal segment indicated in italics originates from the expression and purification tag; it was present during the NMR measurements, but is not part of the TM1081 protein and is not shown in (a) and (b). Underlined residues were identified as being conserved in anti-σ factor antagonists (see text) in a sequence-homology search by BLAST and were subsequently confirmed using the ConSurf server (Ashkenazy et al., 2010 ▶). Black dots indicate residues for which no backbone amide resonances were observed in the 2D [15N,1H]-HSQC spectrum. Above the sequence, cyan arrows indicate the positions of the β-strands and red bars those of the α-helices in the NMR structure.