Table 1. Determination of the NMR structure, a reference crystal structure and a reference NMR structure of the protein TM1081: input for the structure calculations and characterization of bundles of 20 energy-minimized CYANA conformers representing the structures.
Except for the top six entries and the Ramachandran plot statistics, average values and standard deviations for the 20 conformers are given.
| NMR structure† | Reference crystal structure‡ | Reference NMR structure§ | |
|---|---|---|---|
| NOE upper distance limits | 2316 | 4735 | 4055 |
| Intra-residual | 555 | 1035 | 1209 |
| Short-range | 603 | 1112 | 1075 |
| Medium-range | 554 | 1169 | 955 |
| Long-range | 604 | 1419 | 816 |
| Dihedral angle constraints | 423 | 413 | 447 |
| Residual target-function value (Å2) | 2.53 ± 0.29 | 1.17 ± 0.27 | 1.86 ± 0.27 |
| Residual NOE violations | |||
| No. ≥ 0.1 Å | 47 ± 7 | 6 ± 2 | 6 ±3 |
| Maximum (Å) | 0.15 ± 0.01 | 0.14 ± 0.02 | 0.15 ± 0.06 |
| Residual dihedral angle violations | |||
| No. ≥ 2.5° | 0 ± 0 | 1 ± 1 | 2 ± 1 |
| Maximum (°) | 2.16 ± 0.81 | 3.45 ± 1.09 | 3.86 ± 1.37 |
| AMBER energies (kcal mol−1¶) | |||
| Total | −4316 ± 121 | −4323 ± 92 | −4327 ± 85 |
| van der Waals | −317 ± 15 | −431 ± 18 | −333 ± 11 |
| Electrostatic | −5138 ± 107 | −4720 ± 55 | −4992 ± 98 |
| R.m.s.d. from mean coordinates†† (Å) | |||
| Backbone (3–110) | 0.61 ± 0.08 | 0.37 ± 0.06 | 0.59 ± 0.07 |
| All heavy atoms (3–110) | 1.03 ± 0.08 | 0.71 ± 0.07 | 0.98 ± 0.08 |
Backbone (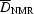 ≤ 0.50 Å) ≤ 0.50 Å) |
0.39 ± 0.07 | 0.31 ± 0.05 | 0.35 ± 0.05 |
All heavy atoms (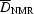 ≤ 0.50 Å) ≤ 0.50 Å) |
0.77 ± 0.06 | 0.59 ± 0.06 | 0.78 ± 0.06 |
| Ramachandran plot statistics‡‡ | |||
| Most favored regions (%) | 71.4 | 84.8 | 75.3 |
| Additional allowed regions (%) | 23.8 | 14.7 | 22.6 |
| Generously allowed regions (%) | 3 | 0.5 | 2.4 |
| Disallowed regions (%) | 1.7 | 0.0 | 0.7 |
Structure calculated from the experimental NMR data. The top six entries represent the input generated in the final cycle of the ATNOS/CANDID and CYANA calculation.
Structure calculated with CYANA from conformational constraints derived from the molecular model representing the crystal structure and subjected to the same energy minimization as the experimental NMR structure (Jaudzems et al., 2010 ▶).
Structure calculated with CYANA from conformational constraints derived from the bundle of 20 molecular models representing the NMR structure and subjected to the same energy minimization as the experimental NMR structure (Jaudzems et al., 2010 ▶).
1 cal = 4.186 J.
The numbers in parentheses indicate the residues for which the r.m.s.d. was calculated. Residues with 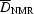 ≤ 0.50 Å are identified in Fig. 1 ▶(c).
≤ 0.50 Å are identified in Fig. 1 ▶(c).
