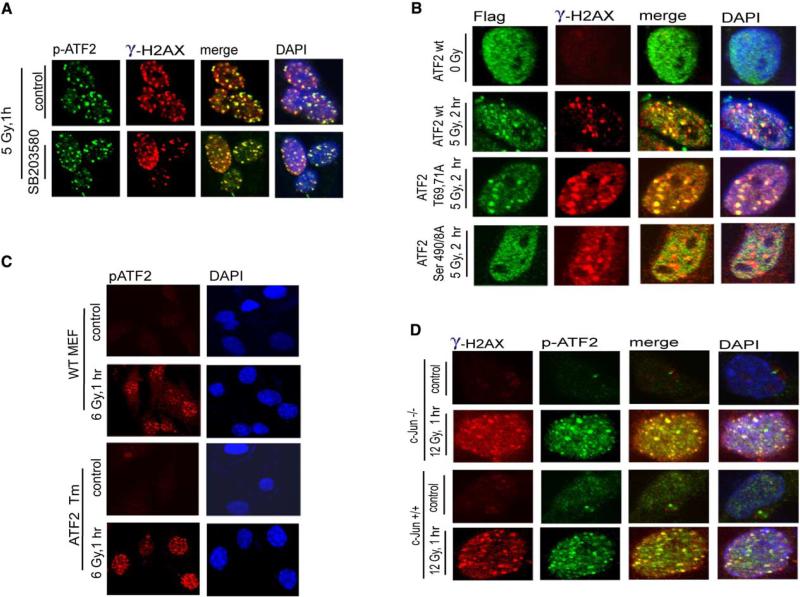
Figure 5. ATF2 Role in the DNA Damage Response Is Uncoupled from Its Transcriptional Activity.
(A) ATF2 phosphorylation by JNK/p38 is not required for recruitment to DSB repair foci after IR. A pharmacological inhibitor of p38/JNK (SB203580, 10 μM, which is sufficient for inhibition of both JNK and p38 kinases) was added to MeWo cells 2 hr before mock treatment or IR (5 Gy). The inhibitor was kept in the medium for 1 hr after treatment, when cells were fixed and subjected to analysis with antibodies to γ-H2AX and p-ATF2.
(B) Colocalization of transcriptionally inactive ATF2 with γ-H2AX in IRIF following IR. ATF2 forms (wt, transcriptionally inactive [T69,71A], and ATM phosphomutants [S490/A] were transfected into MeWo cells that were inhibited for ATF2 expression with corresponding RNAi. Immunostaining of the exogenous forms of ATF2 was carried out 2.5 hr after IR using antibodies to the Flag tag (green). Cells were also analyzed using antibodies to γ-H2AX (red).
(C) ATF2 lacking the DNA binding domain is localized to DSB-induced foci. ATF2 mutant cells expressing a transcriptionally inactive form of ATF2 that lacks DNA binding and part of the leucine zipper domains were subjected to mock or IR treatment (6 Gy) and analyzed to detect ATF2 localization in DSB-induced foci using p-ATF2 antibodies.
(D) ATF2 foci are formed in IR-treated c-Jun−/− cells. Cells lacking c-Jun were irradiated (12 Gy) and fixed 1 hr later for immunostaining using p-ATF2 and γ-H2AX antibodies.