Abstract
Objective
The aims of this study were to estimate the dose to radiosensitive organs (glandular breast and lung) in patients of various sizes undergoing routine chest CT examinations with and without tube current modulation; to quantify the effect of tube current modulation on organ dose; and to investigate the relation between patient size and organ dose to breast and lung resulting from chest CT examinations.
Materials and Methods
Thirty voxelized models generated from images of patients were extended to include lung contours and were used to represent a cohort of women of various sizes. Monte Carlo simulation–based virtual MDCT scanners had been used in a previous study to estimate breast dose from simulations of a fixed-tube-current and a tube current–modulated chest CT examinations of each patient model. In this study, lung doses were estimated for each simulated examination, and the percentage organ dose reduction attributed to tube current modulation was correlated with patient size for both glandular breast and lung tissues.
Results
The average radiation dose to lung tissue from a chest CT scan obtained with fixed tube current was 23 mGy. The use of tube current modulation reduced the lung dose an average of 16%. Reductions in organ dose (up to 56% for lung) due to tube current modulation were more substantial among smaller patients than larger. For some larger patients, use of tube current modulation for chest CT resulted in an increase in organ dose to the lung as high as 33%. For chest CT, lung dose and breast dose estimates had similar correlations with patient size. On average the two organs receive approximately the same dose effects from tube current modulation.
Conclusion
The dose to radiosensitive organs during fixed-tube-current and tube current–modulated chest CT can be estimated on the basis of patient size. Organ dose generally decreases with the use of tube current–modulated acquisition, but patient size can directly affect the dose reduction achieved.
Keywords: CT, Monte Carlo simulation, radiation dose, tube current modulation, voxelized patient model
CT is unique from other imaging techniques in that 3D image acquisition of the entire thoracic region can be performed within a single breath-hold. As a result, chest CT is frequently used as a diagnostic tool. The news media have been giving increased attention to exposure of the general population to ionizing radiation during medical imaging, specifically CT [1]. For chest CT, the most radiosensitive organs are the lungs and glandular breast [2–4]. Risk of breast cancer may correlate with doses less than 100 mGy, and risk of lung cancer may correlate with doses as low as 100 mGy [5].
In discussion of individual risk during CT, organ dose is a better measure for estimating patient risk than is effective dose because effective dose is intended for estimating radiation exposure of entire populations, not for individual dose estimates [6, 7]. Most existing methods for estimating radiation dose to the radiosensitive organs during chest CT are based on measurements or simulations of a single patient model or phantom with unrealistic anatomic features (e.g., breasts modeled as a homogeneous material located directly anterior to the thoracic region) [2, 8–11]. It is not known how well these methods serve for estimation of organ doses in an actual patient population, which includes natural variations in patient size and breast composition.
The primary limitation of most previous tools used for estimation of organ dose during chest CT may be that those methods do not account for the effects of tube current modulation. Tube current modulation is an acquisition technique often used in CT scanners to decrease the overall dose to the patient while maintaining image quality [12, 13]. Tube current modulation is accomplished by reducing the product of the tube current in milliamperes and the time in seconds for regions of lower attenuation, such as the lungs, and increasing the tube current–time product for regions of higher attenuation, such as the shoulders [14–16]. In more recent algorithms, tube current is modulated in three dimensions, accounting for the elliptic shape of a patient and for changes in attenuation, such as across the shoulder–lung interface [17, 18]. The most common method of comparing the doses of specific CT acquisitions is to measure the CT dose index (CTDI), but CTDI is not defined, and cannot be measured, for tube current–modulated CT acquisition.
Many imaging centers use tube current–modulated CT acquisition to decrease radiation exposure to patients, but it is not known how the modulation affects dose absorption in the patient. Published estimates of dose reduction with tube current modulation are generally based on a comparison of the total tube current–time product of a modulated examination and the total tube current–time product of a fixed-tube-current examination. In those studies [19–22] the estimated dose savings of tube current modulation in chest CT have been between 17% and 43% for 3D modulation. These dose estimates do not directly establish how radiation dose to radiosensitive organs is affected by tube current–modulated acquisition. They simply are estimates of the decrease in tube current output. Vollmar and Kalender [23] estimated that the actual breast dose reduction from tube current modulation is 10%; they did not assess lung dose. Those investigators estimated breast dose by measuring and simulating breast dose reduction in a semianthropomorphic phantom that was homogeneous along the z-axis.
Using Monte Carlo simulation–based MDCT scanner models, we can overcome the limitations of previous dose estimation tools by incorporating a range of actual anatomic features of patients undergoing patient-specific tube current–modulated CT acquisition and by incorporating the actual scanner spectra, geometries, and tube current modulation schemes [24–26]. The purposes of this study were to extend previous work with detailed Monte Carlo simulations to estimate the radiation dose to radiosensitive lung tissue in patients of various sizes undergoing routine chest CT examinations with and without tube current modulation; to quantify the effect of tube current modulation on organ dose; and to investigate the relation between patient size and organ dose to the breast and lung during chest CT examinations.
Materials and Methods
The simulations used in this study include previously validated virtual CT scanners that take into account details of the scanner, including helical source path, actual source spectra, filtration, and geometry [24–26]. The methods used to simulate tube current modulation were those reported previously [27]. Voxelized patient models based on CT images of actual women were extended to include lung contours for this study. Monte Carlo simulations of chest CT examinations were performed on these voxelized patient models. Radiation dose was estimated for the lung tissue of each patient model for both fixed-tube-current and tube current–modulated chest CT examinations and compared with similar, previously obtained estimates for breast tissue.
Patient Cohort and Image Data
Thirty detailed voxelized models representing the anatomic features of women 16–89 years old were used [27]. These models were based on patient images from a cohort of 45 women who had undergone clinically indicated tube current–modulated CT of the thoracic region. All of the patient models were based on chest CT examinations performed with attenuation-based 3D tube current modulation (CareDose4D, Siemens Healthcare). The specific clinical indication for each examination was not available for the patient images, from which identifying information had been removed. The original tube current modulation scheme and reference tube current–time products were obtained for each examination. Fifteen of the 45 CT examinations were not used because of technical complications such as problems retrieving tube current modulation data from the scanner. For this HIPAA-compliant study, institutional review board approval was obtained for the use of the CT images, from which identifying information had been removed. Each voxelized patient model included at least the anatomic structures from the thoracic inlet to the lung bases. The perimeter (or circumference) of each patient at the skin–air boundary was included in this study as an indicator of patient size. The perimeter was measured on one transverse image with the techniques described in Angel et al. [27].
The simulated tube current–modulated examination of each patient model matched the acquisition protocol from the patient's original examination. Images of nine of the patient models were originally acquired with a 16-MDCT scanner (Sensation 16, Siemens Healthcare) at a nominal beam collimation of 16 × 0.75 mm, 120 kVp, rotation time of 0.42–0.5 seconds, and pitch of 0.65–1.0. Images of the other 21 patient models were acquired with a 64-MDCT scanner (Sensation 64, Siemens Healthcare) at a nominal beam collimation of 32 × 0.6 mm (n = 20) or 24 × 1.2 mm (n = 1), 120 kVp, rotation time of 0.5 seconds, and pitch of 0.8–1.0. All patient images were reconstructed with a B30f reconstruction kernel and contiguous images with a thickness of 2 or 3 mm. The 16-MDCT scanner tube current modulation algorithm was set to the weak tube current–time product adaptation for slim patients and to the average tube current–time product adaptation for larger patients. The 64-MDCT scanner tube current modulation algorithm was set to the average tube current–time product adaptation for slim and large patients.
Extension of Patient Models
Voxelized models of the anatomic features of each patient were obtained from previous work [27] and extended to include lung-tissue contours. The lung contours were semiautomatically segmented with a method in which thresholds were dynamically selected for edge detection [28]. The voxels within the contoured lungs were modeled as lung tissue [29] with density ranging from 0.048 to 0.65 g/cm3 on the basis of the attenuation of the lung in the original image data. This process resulted in 30 voxelized models with both lung and breast tissue segmented. These models were used in the Monte Carlo CT simulations.
MDCT Source Models
Monte Carlo simulations were used to simulate CT scans by means of modeling of the voxelized patients, scanner geometry, and photon transport through the voxelized patients according to a method described previously [27]. All simulations were made with MCNPX version 2.5.c software [30, 31]. Two virtual MDCT scanners representing the Sensation 16 and Sensation 64 (Siemens Healthcare) models were obtained [24–26]. These virtual scanners include the x-ray spectrum, filtration, and geometry of the specific scanner model and were found to agree with physical measurement results from standardized dosimetric phantoms (CTDI phantoms) to within 10% [24–27].
Modeling of Tube Current Modulation
For each patient, the actual tube current modulation values used in the patient's clinical scan were obtained from the raw projection data according to the method described previously [27]. This modulation scheme, which is illustrated in Figure 1, includes the table position, gantry angle, and tube current used for each projection as the tube rotates around and traverses the patient. The tube current values were used as weighting factors for each projection in the Monte Carlo simulations of a CT scan for the corresponding patient model. Using the exact tube current modulation schema allows accurate representation of the tube current modulation specific to each patient.
Fig. 1.
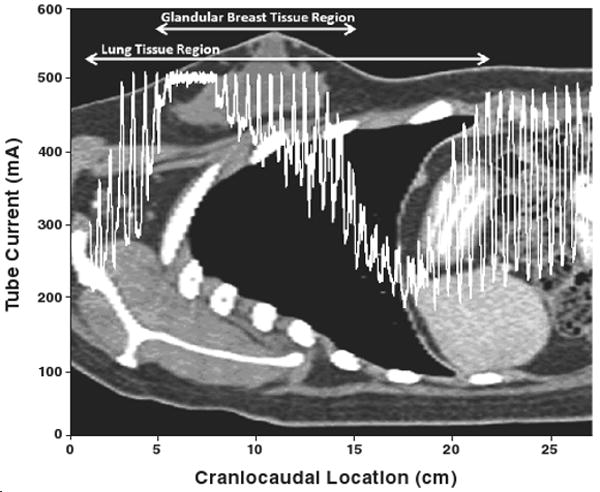
28-year-old woman undergoing tube current modulated chest CT. Plot shows patient's tube current modulation scheme overlaid on sagittal view of patient. Tube current versus table location is shown for patient model with perimeter of 101 cm.
Simulated Chest CT Scans
Two scan protocols were simulated for each voxelized patient model: the actual tube current–modulated scan and a fixed-tube-current scan [27]. With the exception of tube current, the simulated acquisition protocols (e.g., collimation, rotation time, pitch) were consistent between the two simulated examinations and matched those used for the actual patient's chest CT examination. A Monte Carlo simulation tally was used to calculate the average radiation dose, in milligrays, to the lung tissues [24, 25, 27, 30–32].
The lung dose saving from tube current modulation was determined by calculation of the percentage reduction of lung dose between the tube current–modulated and fixed-tube-current acquisitions. This value was calculated for each patient and was recorded with patient perimeter for study of the effects of patient size on organ dose. To allow comparison of a peripheral organ to a large central organ, previous breast dose estimates [27] were presented alongside the lung dose estimates. Linear regression analysis was performed on both lung and breast dose to assess the correlation between organ dose and patient perimeter, and p was calculated to determine statistical significance. A value of p < 0.5 indicated a significant difference.
Results
Table 1 shows the patient perimeters and ages, previously reported breast dose estimates [27], and the lung dose results for the simulated scans performed on each patient model. The average lung dose at fixed-tube-current chest CT was 23 mGy (range, 14–34 mGy). The average lung dose at tube current–modulated chest CT was 18 mGy (range, 12–25 mGy). The average lung dose reduction from tube current modulation was 16% with a maximum dose decrease of 56% and a maximum dose increase (dose penalty) of 33%.
TABLE 1. Results of Simulated Scans.
| Breast Dose (mGy)a | Lung Dose (mGy) | ||||||
|---|---|---|---|---|---|---|---|
| Patient Perimeter (cm) | Patient Age (y) | Fixed Tube Current | Tube Current Modulation | Percentage Dose Reduction | Fixed Tube Current | Tube Current Modulation | Percentage Dose Reduction |
| 81 | 63 | 29 | 13 | 57 | 34 | 17 | 49 |
| 87 | 57 | 23 | 8 | 64 | 27 | 12 | 56 |
| 88 | 35 | 22 | 9 | 60 | 26 | 12 | 53 |
| 89 | 64 | 21 | 12 | 41 | 26 | 17 | 36 |
| 89 | 67 | 26 | 13 | 50 | 31 | 16 | 47 |
| 92 | 41 | 24 | 14 | 43 | 31 | 19 | 39 |
| 92 | 16 | 19 | 9 | 52 | 24 | 13 | 44 |
| 93 | 89 | 22 | 8 | 62 | 25 | 12 | 52 |
| 98 | 71 | 16 | 14 | 11 | 22 | 19 | 14 |
| 99 | 61 | 21 | 15 | 27 | 25 | 18 | 27 |
| 100 | 52 | 19 | 13 | 34 | 24 | 14 | 42 |
| 101 | 28 | 22 | 19 | 14 | 24 | 19 | 22 |
| 101 | 78 | 20 | 12 | 40 | 26 | 18 | 30 |
| 112 | 62 | 20 | 18 | 10 | 25 | 25 | 2 |
| 114 | 75 | 16 | 14 | 8 | 17 | 18 | −2 |
| 114 | 70 | 18 | 12 | 34 | 25 | 16 | 39 |
| 115 | 35 | 16 | 11 | 30 | 22 | 17 | 20 |
| 116 | 77 | 18 | 15 | 15 | 22 | 19 | 15 |
| 119 | 71 | 16 | 18 | −12 | 22 | 22 | 0 |
| 121 | 44 | 16 | 15 | 5 | 18 | 18 | 0 |
| 122 | 56 | 17 | 15 | 10 | 21 | 19 | 8 |
| 123 | 49 | 16 | 18 | −12 | 19 | 21 | −10 |
| 124 | 31 | 16 | 19 | −15 | 20 | 22 | −8 |
| 125 | 70 | 19 | 19 | 0 | 22 | 23 | −2 |
| 128 | 39 | 14 | 20 | −41 | 16 | 22 | −33 |
| 128 | 29 | 16 | 18 | −14 | 18 | 20 | −14 |
| 132 | 69 | 15 | 16 | −8 | 17 | 18 | −2 |
| 134 | 62 | 14 | 17 | −21 | 19 | 22 | −16 |
| 135 | 55 | 14 | 16 | −15 | 17 | 19 | −12 |
| 142 | 62 | 14 | 16 | −16 | 14 | 16 | −16 |
| 111 (mean) | 56 | 19 | 15 | 17 | 23 | 18 | 16 |
| 81 (minimum) | 16 | 14 | 8 | -41 | 14 | 12 | -33 |
| 142 (maximum) | 89 | 29 | 20 | 64 | 34 | 25 | 56 |
Breast dose estimates from [27].
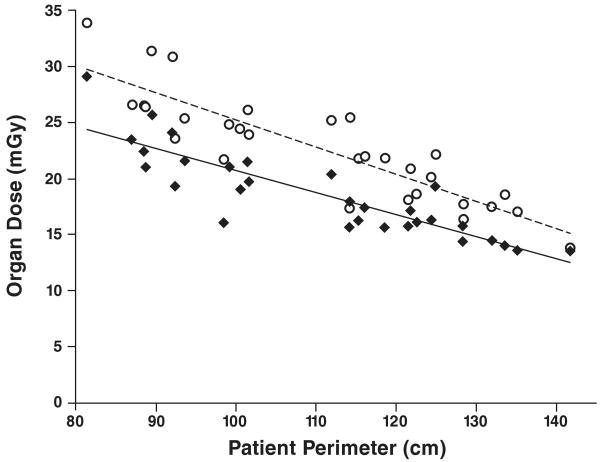
The results for organ dose as a function of patient size (perimeter) at fixed-tube-current chest CT are shown in Figure 2. The relation between patient perimeter and organ dose for fixed tube current chest CT can be represented as: DFbreast = (−0.20 × perimeter) + 40.3 and DFlung = (−0.24 × perimeter) + 49.5, where DF is the organ dose, in milligrays, for fixed-tube-current chest CT. The equations are based on linear regression analysis of the lung dose results from this study and previously acquired breast dose results [27]. These equations showed a significant linear correlation between patient perimeter and breast dose (R2 = 0.76; p < 0.001) and between patient perimeter and lung dose (R2 = 0.77; p < 0.001) at fixed-tube-current chest CT.
Fig. 2.
Graph of results for fixed-tube-current chest CT examination shows correlation between patient perimeter and radiation dose to glandular breast (diamonds) (R2 = 0.76) and lung (circles) (R2 = 0.77).
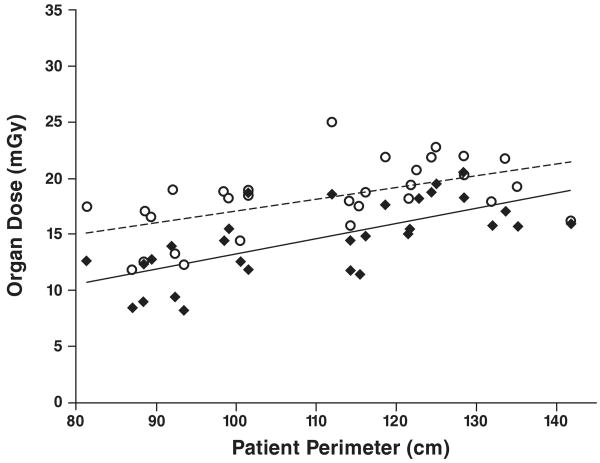
The results for organ dose as a function of patient size for tube current–modulated chest CT are shown in Figure 3. The relation between patient perimeter and organ dose for tube current–modulated chest CT (DTCM) can be represented as DTCMbreast = (0.134 × perimeter) − 0.196 and DTCMlung = (0.104 × perimeter) + 6.60. Linear regression analysis for the tube current–modulated chest CT simulations showed a slope close to 0 with lower coefficients of determination but still showed significant linear correlation between patient perimeter and breast dose (R2 = 0.46; p < 0.001) and between patient perimeter and lung dose (R2 = 0.31; p = 0.001).
Fig. 3.
Graph of results for tube current–modulated chest CT examination shows correlation between patient perimeter and radiation dose to glandular breast (diamonds) (R2 = 0.46) and lung (circles) (R2 = 0.31).
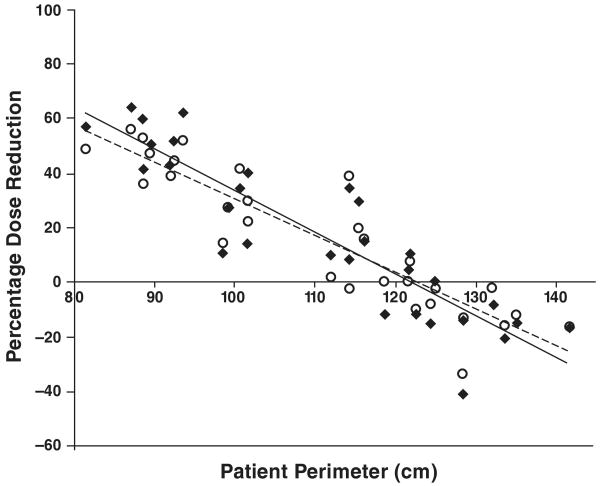
Figure 4 shows percentage decrease in organ dose as a function of patient size with use of tube current–modulated as opposed to fixed-tube-current acquisition for chest CT. In nine of the 30 patient models, tube current modulation caused an increase in organ dose compared with fixed-tube-current acquisition. An increase in organ dose is shown in Figure 4 by a negative percentage dose reduction value. This finding was true for patients with a perimeter greater than 122 cm. As expected, larger women receive less dose reduction from tube current modulation than do smaller women. The relation between patient perimeter and percent dose reduction (PDR) from tube current modulation can be represented for breast and lung as follows: PDRbreast = (−1.53 × perimeter) + 186 and PDRlung = (−1.35 × perimeter) + 165. Linear regression analysis of percent dose reduction showed significant linear correlation between patient perimeter and percent breast dose reduction (R2 = 0.81; p < 0.001) and between patient perimeter and percent lung dose reduction (R2 = 0.82; p = 0.001).
Fig. 4.
Graph shows correlation between patient perimeter and percentage reduction or increase in dose to glandular breast (R2 = 0.81) and lung (R2 = 0.82) with use of tube current modulation as opposed to fixed tube current in chest CT. Negative percentage dose reduction value denotes increase in organ dose with use of tube current modulation as opposed to fixed tube current.
Discussion
In this study, Monte Carlo simulation methods were used to obtain detailed estimates of radiation dose to the lungs of patients undergoing chest CT examinations with and without patient-specific tube current modulation. The patient models represented the actual anatomy of women and included a range of patient sizes. As in a previous study [27], we found that the radiation dose to organs in the scan volume can increase rather decrease with the use of tube current modulation. Patient perimeter can easily be measured in the clinic with a tape measure before an examination. Linear regression analysis showed that we can use linear equations to estimate organ dose on the basis of patient perimeter for fixed-tube-current and tube current–modulated acquisitions and to estimate percentage dose reduction attained with tube current modulation. We found not only that the glandular breast, which is considered a peripheral organ, and lung, which is considered a large medially situated organ, receive similar doses during chest CT, both with and without tube current modulation, but also that the dose reduction from tube current modulation is similar for the two organs, especially with respect to patient size.
The limitations of this study were similar to those in our previous study [27]. Specifically, the patient cohort is not necessarily an accurate represention of the general patient population. In addition, only two scanners were modeled, and only one specific tube current modulation algorithm was evaluated. Results of another study [33] suggest that organ dose estimates during fixed-tube-current examinations can be extrapolated for estimates of organ doses for other scanners through comparison of physical phantom measurements. In future work, we will investigate the robustness of that theory for tube current modulated CT acquisition.
Image quality was not directly measured in this study. However, the value used for the fixed-tube-current simulations was selected to provide a condition that would yield approximately the same image quality for a standard-sized patient as would the tube current modulation scheme. Because in this condition, image quality is adjusted only for a standard-sized patient, there would be no adjustment for patient size in the fixed-tube-current simulations. To have the same image quality as CT of smaller patients, CT of larger patients must be performed with greater tube current. Therefore, although tube current modulation has been found to increase organ dose for larger patients, image quality also can be improved. Some imaging centers adjust fixed tube current settings for patient size. If patient size adjustments had been modeled in this study, the results might have shown less variation with patient size.
Glandular breast and lung are two of the most radiosensitive organs irradiated during routine thoracic CT. Tube current–modulated CT acquisition was found to reduce the radiation dose to these tissues, especially in smaller patients (perimeter < 122 cm). These radiation dose reductions depend on patient size and can result in organ dose reductions up to 64% in our patient population. Larger patients may not receive a decrease in dose with tube current modulation.
Acknowledgments
Funded by grant R01EB004898 from the National Institute of Biomedical Imaging and Bioengineering (NIBIB) and supported by the UCLA Graduate Division Research Mentorship award.
A. N. Primak received partial support from a research grant from Siemens Medical Systems during work on this article and currently is employed by Siemens. E. Angel currently is employed by Toshiba America Medical Systems but did not receive any financial support from Toshiba or any other commercial entities during this research project.
References
- 1.Mettler FA, Jr, Thomadsen BR, Bhargavan M, et al. Medical radiation exposure in the U.S. in 2006: preliminary results. Health Phys. 2008;95:502–507. doi: 10.1097/01.HP.0000326333.42287.a2. [DOI] [PubMed] [Google Scholar]
- 2.Hurwitz LM, Yoshizumi TT, Reiman RE, et al. Radiation dose to the female breast from 16-MDCT body protocols. AJR. 2006;186:1718–1722. doi: 10.2214/AJR.04.1917. [DOI] [PubMed] [Google Scholar]
- 3.Mayo JR, Aldrich J, Muller NL. Radiation exposure at chest CT: a statement of the Fleischner Society. Radiology. 2003;228:15–21. doi: 10.1148/radiol.2281020874. [DOI] [PubMed] [Google Scholar]
- 4.The 2007 recommendations of the International Commission on Radiological Protection: ICRP publication 103. Ann ICRP. 2007;37:1–332. doi: 10.1016/j.icrp.2007.10.003. No authors listed. [DOI] [PubMed] [Google Scholar]
- 5.Preston DL, Ron E, Tokuoka S, et al. Solid cancer incidence in atomic bomb survivors: 1958–1998. Radiat Res. 2007;168:1–64. doi: 10.1667/RR0763.1. [DOI] [PubMed] [Google Scholar]
- 6.Martin CJ. Effective dose: how should it be applied to medical exposures? Br J Radiol. 2007;80:639–647. doi: 10.1259/bjr/25922439. [DOI] [PubMed] [Google Scholar]
- 7.Brenner D, Hall E. Computed tomography: an increasing source of radiation exposure. N Engl J Med. 2007;357:2277–2284. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 8.Parker MS, Hui FK, Camacho MA, Chung JK, Broga DW, Sethi NN. Female breast radiation exposure during CT pulmonary angiography. AJR. 2005;185:1228–1233. doi: 10.2214/AJR.04.0770. [DOI] [PubMed] [Google Scholar]
- 9.CT scanner matching data, tables of CTDI values in air, CTDIw, and phantom factor values. [July 20, 2009]; ImPACT Website. http://www.ImPACTscan.org.
- 10.Jones D, Shrimpton PC. NRPB R-250. Didcot, UK: National Radiological Protection Board; 1991. Survey of the practice in the UK. Part 3. Normalized organ doses calculated using Monte Carlo techniques. [Google Scholar]
- 11.Hurwitz LM, Yoshizumi TT, Goodman PC, et al. Radiation dose savings for adult pulmonary embolus 64-MDCT using bismuth breast shields, lower peak kilovoltage, and automatic tube current modulation. AJR. 2009;192:244–253. doi: 10.2214/AJR.08.1066. [DOI] [PubMed] [Google Scholar]
- 12.McCollough CH, Bruesewitz MR, Kofler JM., Jr CT dose reduction and dose management tools: overview of available options. RadioGraphics. 2006;26:503–512. doi: 10.1148/rg.262055138. [DOI] [PubMed] [Google Scholar]
- 13.Kalra MK, Naz N, Rizzo SM, Blake MA. Computed tomography radiation dose optimization: scanning protocols and clinical applications of automatic exposure control. Curr Probl Diagn Radiol. 2005;34:171–181. doi: 10.1067/j.cpradiol.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Kalender WA, Wolf H, Suess C, Gies M, Greess H, Bautz WA. Dose reduction in CT by on-line tube current control: principles and validation on phantoms and cadavers. Eur Radiol. 1999;9:323–328. doi: 10.1007/s003300050674. [DOI] [PubMed] [Google Scholar]
- 15.Gies M, Kalender WA, Wolf H, Suess C. Dose reduction in CT by anatomically adapted tube current modulation. Part 1. Simulation studies. Med Phys. 1999;26:2235–2247. doi: 10.1118/1.598779. [DOI] [PubMed] [Google Scholar]
- 16.Kalender WA, Wolf H, Suess C. Dose reduction in CT by anatomically adapted tube current modulation. Part 2. Phantom measurements. Med Phys. 1999;26:2248–2253. doi: 10.1118/1.598738. [DOI] [PubMed] [Google Scholar]
- 17.Greess H, Wolf H, Baum U, Kalender WA, Bautz W. Dosage reduction in computed tomography by anatomy-oriented attenuation-based tube-current modulation: the first clinical results [in German] Rofo. 1999;170:246–250. doi: 10.1055/s-2007-1011035. [DOI] [PubMed] [Google Scholar]
- 18.Kalra MK, Maher MM, Toth TL, Kamath RS, Halpern EF, Saini S. Comparison of z-axis automatic tube current modulation technique with fixed tube current CT scanning of abdomen and pelvis. Radiology. 2004;232:347–353. doi: 10.1148/radiol.2322031304. [DOI] [PubMed] [Google Scholar]
- 19.Mulkens TH, Bellinck P, Baeyaert M, et al. Use of an automatic exposure control mechanism for dose optimization in multi-detector row CT examinations: clinical evaluation. Radiology. 2005;237:213–223. doi: 10.1148/radiol.2363041220. [DOI] [PubMed] [Google Scholar]
- 20.Rizzo S, Kalra M, Schmidt B, et al. Comparison of angular and combined automatic tube current modulation techniques with constant tube current CT of the abdomen and pelvis. AJR. 2006;186:673–679. doi: 10.2214/AJR.04.1513. [DOI] [PubMed] [Google Scholar]
- 21.Tack D, De Maertelaer V, Gevenois PA. Dose reduction in multidetector CT using attenuation-based online tube current modulation. AJR. 2003;181:331–334. doi: 10.2214/ajr.181.2.1810331. [DOI] [PubMed] [Google Scholar]
- 22.Kalra MK, Rizzo S, Maher MM, et al. Chest CT performed with z-axis modulation: scanning protocol and radiation dose. Radiology. 2005;237:303–308. doi: 10.1148/radiol.2371041227. [DOI] [PubMed] [Google Scholar]
- 23.Vollmar SV, Kalender WA. Reduction of dose to the female breast in thoracic CT: a comparison of standard-protocol, bismuth-shielded, partial and tube-current-modulated CT examinations. Eur Radiol. 2008;18:1674–1682. doi: 10.1007/s00330-008-0934-9. [DOI] [PubMed] [Google Scholar]
- 24.Jarry G, DeMarco JJ, Beifuss U, Cagnon CH, McNitt-Gray MF. A Monte Carlo–based method to estimate radiation dose from spiral CT: from phantom testing to patient-specific models. Phys Med Biol. 2003;48:2645–2663. doi: 10.1088/0031-9155/48/16/306. [DOI] [PubMed] [Google Scholar]
- 25.DeMarco JJ, Cagnon CH, Cody DD, et al. A Monte Carlo based method to estimate radiation dose from multidetector CT (MDCT): cylindrical and anthropomorphic phantoms. Phys Med Biol. 2005;50:3989–4004. doi: 10.1088/0031-9155/50/17/005. [DOI] [PubMed] [Google Scholar]
- 26.DeMarco JJ, Cagnon CH, Cody DD, et al. Estimating radiation doses from multidetector CT using Monte Carlo simulations: effects of different size voxelized patient models on magnitudes of organ and effective dose. Phys Med Biol. 2007;52:2583–2597. doi: 10.1088/0031-9155/52/9/017. [DOI] [PubMed] [Google Scholar]
- 27.Angel E, Yaghmai N, Jude CM, et al. Monte Carlo simulations to assess the effects of tube current modulation on breast dose for multidetector CT. Phys Med Biol. 2009;54:497–512. doi: 10.1088/0031-9155/54/3/003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tran LN, Brown MS, Goldin JG, et al. Comparison of treatment response classifications between unidimensional, bidimensional, and volumetric measurements of metastatic lung lesions on chest CT. Acad Radiol. 2004;11:1355–1360. doi: 10.1016/j.acra.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 29.International Council on Radiation Utilization. Tissue substitutes in radiation dosimetry and measurement: report 44 of the International Commission on Radiation Units and Measurements. Bethesda, MD: International Council on Radiation Utilization (ICRU); 1989. [Google Scholar]
- 30.Waters E. MCNPX user's manual version 2.4.0: Los Alamos National Laboratory report LA-CP-02-408. Los Alamos, NM: Los Alamos National Laboratory; 2002. [Google Scholar]
- 31.Waters E. MCNPX version 2.5.C: Los Alamos National Laboratory report LA-UR-03-2202. Los Alamos, NM: Los Alamos National Laboratory; 2003. [Google Scholar]
- 32.Hubbell JH. Tables of x-ray mass absorption coefficients and mass energy-absorption coefficients (version 1.03) Gaithersburg, MD: National Institute of Standards and Technology; 1995. [July 20, 2009]. http://physics.nist.gov. [Google Scholar]
- 33.Turner A, Angel E, Zhang D, et al. RSNA 2008. Oak Brook, IL: Radiological Society of North America; 2008. [July 20, 2009]. Comparison of organ dose among 64 detector MDCT scanners from different manufacturers: a Monte Carlo simulation study (abstr) http://rsna2008.rsna.org/event_display.cfm?em_id=6019484. [Google Scholar]