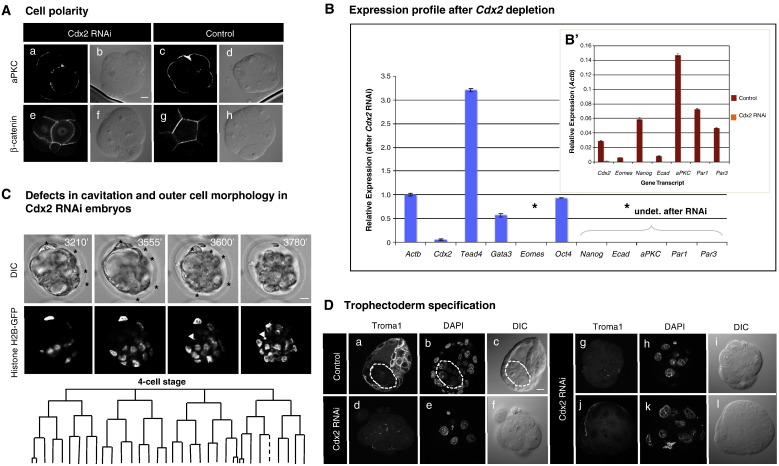
Fig. 4.
Cdx2 depletion from the zygote stage affects cell polarisation, outer cell morphology and expression of trophectoderm-marker genes. (A) To observe effect on cell polarisation, Cdx2-depleted zygotes were fixed at the 8-cell stage and immuno-stained for aPKC or β-catenin. Comparison of expression level of these factors in Cdx2-depleted (left) and a control embryo (right) are shown. For each factor, images were taken using the same laser settings and phase images are also shown. Note decreased expression of apically localised aPKC protein and increased β-catenin in the nucleus, in the Cdx2-depleted embryos. Scale bar 10 μm. (B) Quantitative real-time PCR analysis of trophectoderm (Cdx2, Tead4, Gata3 and Eomes), pluripotency-related (Oct4 and Nanog) and polarity-related (Ecad, aPKC, Par1 and Par3) gene mRNA levels at the mid 16-cell stage after Cdx2-specific RNAi from zygote stage (normalised to Actb levels). Expression levels are shown as fold change, comparing control embryos (injected with DsRed mRNA alone) with Cdx2-depleted embryos (injected with Cdx2-specific dsRNA and DsRed mRNA). Errors equal SEM of triplicates. Highlighted transcripts (*) denote those whose expression was reduced to undetectable levels after Cdx2 RNAi. Accordingly, the mRNA expression levels of these genes in control embryos (relative to that of Actb) are shown in the B′ panel to confirm the primers used. (C) Even in Cdx2-depleted embryos that developed beyond 16-cell stage, outer cells morphology was changed and cavitation affected. Representative time-lapse DIC (upper panels) and GFP images (middle panels) of an embryo undergoing cavitation are shown; scale bar 10 μm. Lineage tree for the same embryo generated using SIMI Biocell software (lower panel). White arrowhead on the GFP images and dashed branch of the lineage tree indicate cell death; time of images in minutes relative to 8-cell stage entry is shown. Black stars on DIC images highlight outer cells with abnormal (rounded) morphology prior to cavity collapse (last image of the sequence). (D) Expression of trophectoderm-specific cytokeratins recognised by Troma1 antibody is dramatically reduced after Cdx2 depletion. Immuno-fluorescence staining for Troma1 antigen in representative control blastocysts (panels ‘a–c’—dashed line outlines ICM) and Cdx2-depleted embryos, at a blastocyst equivalent stage (panels ‘d–l’) are shown. DNA DAPI counter-stain and phase images are shown for reference; scale bar 10 µM.