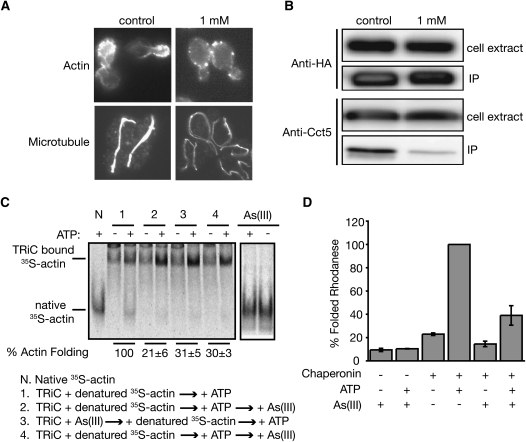
Figure 4.—
As(III) inhibits TRiC functions. (A) Wild-type yeast (BY4743) cells were grown in liquid YPD either in the absence or presence of 1 mm of As(III) for 3 hr. Actin was stained with rhodamine-phalloidin and microtubules were visualized with an anti-α–tubulin antibody. (B) ATP-depleted cell lysates prepared from yeast cells expressing Cdc55–3HA grown in liquid YPD that either contained or lacked 1 mm As(III) were subjected to immunoprecipitation with anti-HA antibody. Western blots of the total cell extracts (5μg) and the immunoprecipitates (IP) from lysates containing 200 μg total protein were analyzed with an anti-HA antibody and an antibody against one of the TRiC subunits Cct5. (C) In vitro binding and folding of denatured [35S]-actin by bovine TRiC both in the presence and absence of 1 mm As(III) were assessed by native gel analysis followed by autoradiography. Three (2, 3, and 4) different schemes of arsenic treatment were tested. Native [35S]-actin samples incubated with or without As(III) were included as controls (right two lanes). The extent of As(III) inhibition in each condition was quantified for three independent experiments and expressed as percentage of actin folding relative to the untreated control. (D) In vitro rhodanese folding by the M. maripaludis TRiC-like chaperonin (Mm-Cpn) was assessed in the presence and absence of 1 mm sodium arsenite as described (Kusmierczyk and Martin 2003).