Figure 1.
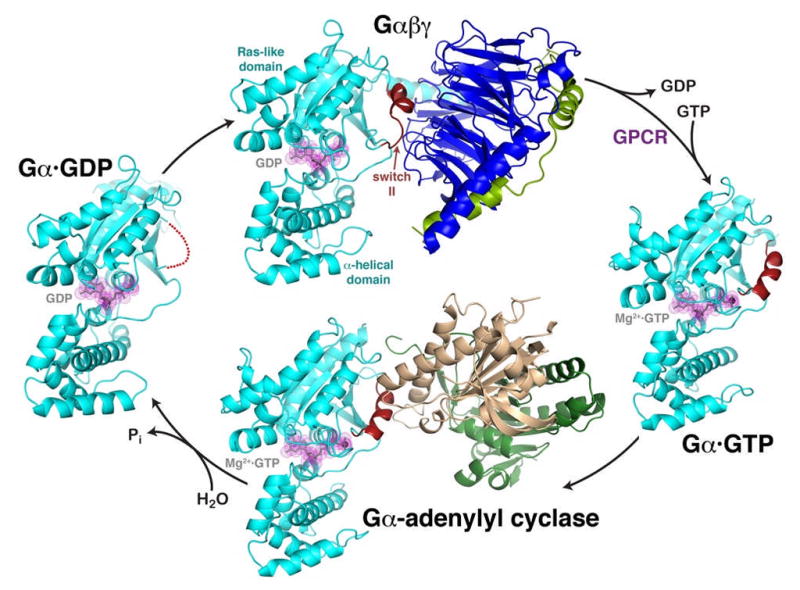
The heterotrimeric G protein cycle.
The inactive Gαβγ heterotrimer (top) is composed of two principal elements, Gα·GDP (cyan, with GDP shown in grey with magenta dot surface) and the Gβγ heterodimer (blue and green). Gβγ sequesters the switch II element (red) such that it is unable to interact with effectors. Activated GPCRs catalyze the release of GDP from Gα, allowing GTP to bind and liberate the activated Gα·GTP subunit (right). In this state, switch II forms a helix stabilized by the γ-phosphate of GTP. The activated Gαsubunit can then interact with effectors, such as the catalytic domains of adenylyl cyclase (bottom, gold and dark green). Switch II forms a major component of the interface, as it does in other characterized effector complexes. The Gαsubunit has a slow GTPase activity that converts GTP to GDP, weakening its interactions with effectors and allowing it to dissociate as a deactivated Gα·GDP subunit (left). In this state, switch II is disordered (red dotted line), and the protein has high affinity for Gβγ subunits, completing the cycle. The structures shown correspond to PDB entries 1GG2 (ref. 27) (top, cyan subunit corresponds to Gαi), 1AZT28 (right, Gαs), 1AZS4 (bottom, Gαs) and 1GDD29 (left, Gαi).
