Figure 2.
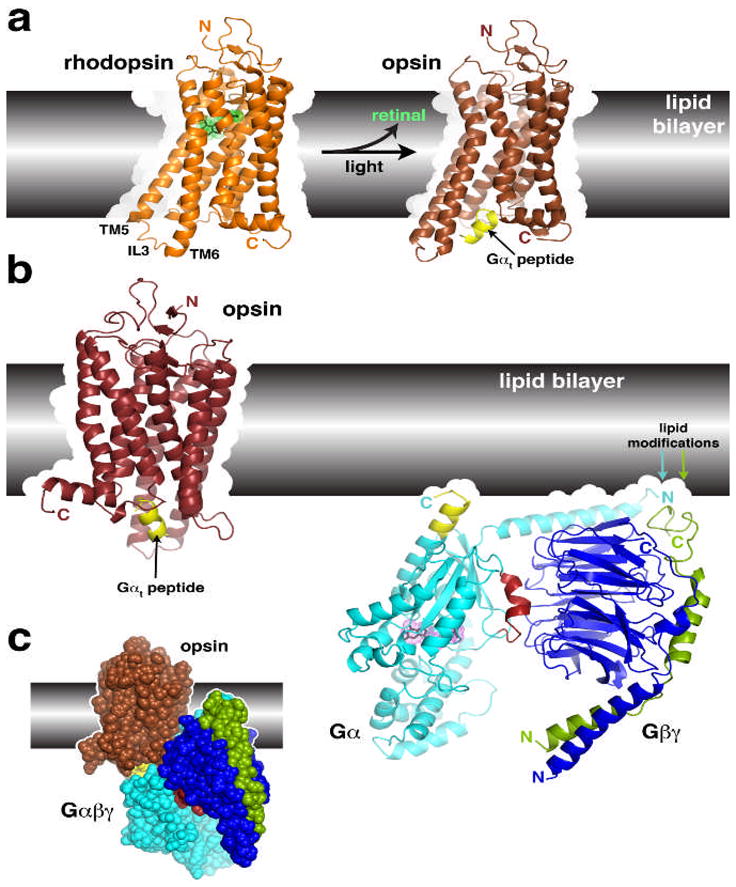
Structural transitions of GPCRs and their interactions with heterotrimeric G proteins.
(a) Comparison of the structures of rhodopsin and opsin. Opsin represents a retinal-depleted form of the GPCR with low basal activity yet shows structural features consistent with changes predicted to occur during rhodopsin activation, such as the outward twist of the third intracellular loop (IL3). Opsin can also be crystallized in complex with a peptide derived from the C-terminus of Gαt (yellow), the region of the heterotrimer most strongly linked to receptor recognition. Retinal is shown as a stick model with a green dot surface. Structures correspond to PDB entries 1GZM30 and 3DQB17. (b) Conceptual problems in docking current models of GPCRs with their heterotrimeric G protein substrates. Here we show the inactive Gαiβ1γ2 complex (PDB 1GG2 (ref. 27)), in which the C-terminus of Gαi was extended according to PDB entry 1AGR31 and the C-terminus of Gγ according to PDB entry 1OMW32. The C-terminal span of Gαi analogous to the Gαt peptide bound to opsin is colored yellow. Comparing the orientation of this helix in each model reveals an an apparent docking incompatibility, because the intact heterotrimer would have to be rotated, roughly counterclockwise, up into the plane of the lipid bilayer in order to superimpose these elements. (c) Collision of Gαβγ with the lipid bilayer when superimposed with the Gαt peptide bound to opsin. The collision suggests that either the model of opsin does not represent a GPCR in a fully activated state, or the G protein heterotrimer must undergo a significant conformational change, or both. A large conformational change is expected in Gαβγ because its interaction with receptors must induce nucleotide release.
