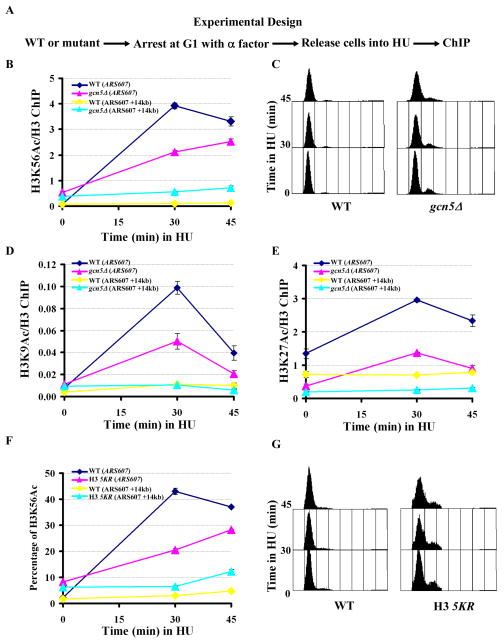
Figure 6.
Gcn5 and five lysine residues at the N-terminus of H3 are required for efficient deposition of newly-synthesized H3 onto replicating DNA. (A) A schematic diagram showing ChIP experimental design. Wild-type or mutant cells (gcn5Δ or H3 5KR) were arrested in G1 using α-factor and then released into HU. At different time points, samples were removed for FACS analysis and ChIP assays using antibodies against H3 or H3K56Ac. (B) The deposition of H3K56Ac onto replicating DNA is compromised in gcn5Δ mutant cells. The ChIP DNA was analyzed using primers that amplify the replication origin ARS607 or a fragment 14 kb away from ARS607 (ARS607+14 kb). The ratio of H3K56Ac ChIP signal over that of H3 was calculated. (C) FACS analysis of the DNA content of wild-type and gcn5Δ mutant cells used in B. (D–E) H3K9Ac (D) and H3K27Ac (E) were detected at replicating DNA. The experiment was performed as described in A and B except that antibodies against H3K9Ac or H3K27Ac were used. (F) Deposition of H3K56Ac was compromised in cells expressing the H3 5KR mutant cells. The experiment was performed as described in A and B except that the percentage of DNA that was precipitated by antibodies against H3K56Ac was calculated by analyzing both ChIP DNA and DNA from whole cells using real-time PCR. (G) FACS analysis of the DNA content of yeast strains used in experiments described in F. Each ChIP experiment was performed independently at least twice. The data represent mean ± SEM of three real-time PCR samples from one experiment. See also Figure S4.