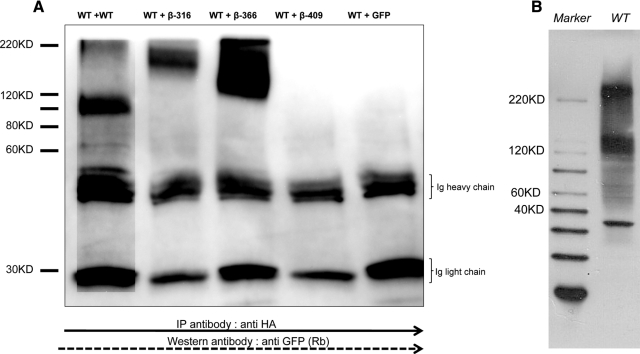
Figure 5.
Coimmunoprecipitation showing the interaction between the WT receptor and β-subunits. A, To study the potential interaction between WT TSH and the TSHR subunit constructs, we cotransfected HA-TSHR and each of the truncated β-subunit constructs. We used a TSHR-GFP vector along with HA-TSHR in a 1:1 ratio in HEK-293 cells as the positive control and a control DNA-GFP vector as a negative control. The cells were harvested after 48 h, and solubilized receptors were prepared as described in Materials and Methods. Individual preparations of protein were initially precleaned with protein G sepharose and then precipitated with anti-HA (2 μg/ml) for 3 h at 4C. This was followed by precipitation of the complex by sepharose G beads for another 2 h. The precipitate was then run on reduced gel and after transfer probed with anti-GFP. The lanes are as indicated: WT+WT, HA-TSHR cotransfected with TSHR-GFP; WT + β-316, WT + β-366, WT + β-409 and WT+ GFP vector. The WT+WT lane showed the presence of a small quanitity of oligomeric forms (>200 kDa) in addition to the glycosylated monomeric subunit of 120 kDa, whereas in the β-316 and β-366 subunit transfected cells we observed exclusively higher order forms of 145–200 kDa, which were resistant to reduction. We did not observe any higher order forms in the β-409 cells because these were solubilized membrane preparations in which this construct failed to express. The control transfected cells lacked the presence of any higher order forms. B, An immunoblot of the WT TSHR protein indicating the clear presence of oligomers. IP, Immunoprecipitation; KD, kilodalton; Rb, Rabit antibody.