Figure 2.
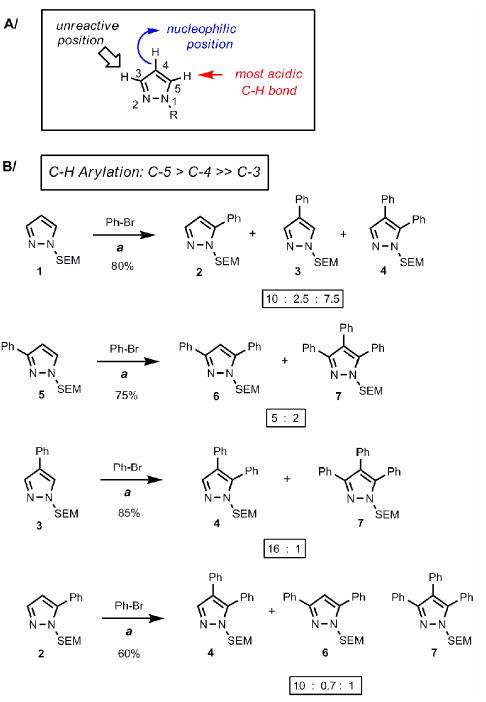
A/ General reactivity properties of pyrazole. B/ Reactivity profile of pyrazoles toward palladium-catalyzed C-H arylation. The C-5 position exhibits the highest reactivity. (a) Reaction conditions: Pyrazole, PhBr (1.5 equiv), Pd(OAc)2 (5 mol %), P(n-Bu)Ad2 (7.5 mol %), K2CO3 (3 equiv), HOPiv (25 mol %), 2.5 M DMA, 140 °C for 12 hours. Isolated yields are shown except for substrate 1, where the substrate conversion and the product ratio was determined by 1H NMR of the crude mixture. All product ratios were confirmed by 1H NMR of crude mixtures.
