Scheme 4.
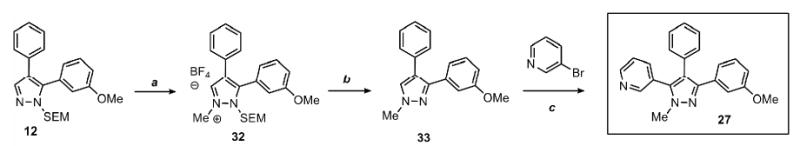
Sequential C-arylation and N-methylation provides a rapid access to 1-methyl-3,4,5-triarylpyrazoles with complete regioselectivity control
Conditions: (a) Pyrazole, Me3O-BF4 (1.2 equiv), CH2Cl2, RT, 1 hr. (b) 3N HCl, EtOH, reflux, 1 h; 70% yield over 2 steps. (c) Pyrazole, ArBr (1.5 equiv), Pd(OAc)2 (5 mol %), P(n-Bu)Ad2 (7.5 mol %), K2CO3 (3 equiv), HOPiv (25 mol %), 2.5 M DMA, 140 °C for 12 hours; 70% yield. Yields are an average of at least two separate isolated yields.
