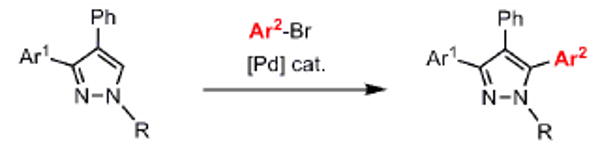
Table 3.
C-arylation of diarylpyrazoles. Substrate scope.
 | ||
|---|---|---|
| Entry | Product | Isolated Yield |
| 1 | 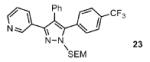 |
77% |
| 2 | 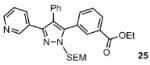 |
88% |
| 3 | 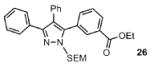 |
64% |
| 4 | 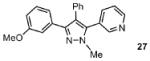 |
70% |
| 5 | 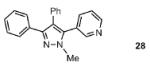 |
67% |
| 6 | 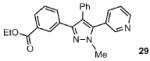 |
53% |
| 7 | 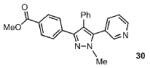 |
54% |
| 8 | 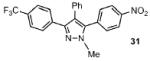 |
43% |
Reaction conditions: Pyrazole, ArBr (1.5 equiv), Pd(OAc)2 (5 mol %), P(n-Bu)Ad2 (7.5 mol %), K2CO3 (3 equiv), HOPiv (25 mol %), 2.5 M DMA, 140 °C, 12 hours. Yields are an average of at least two separate isolated yields.
