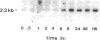Abstract
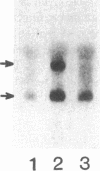
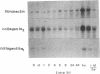
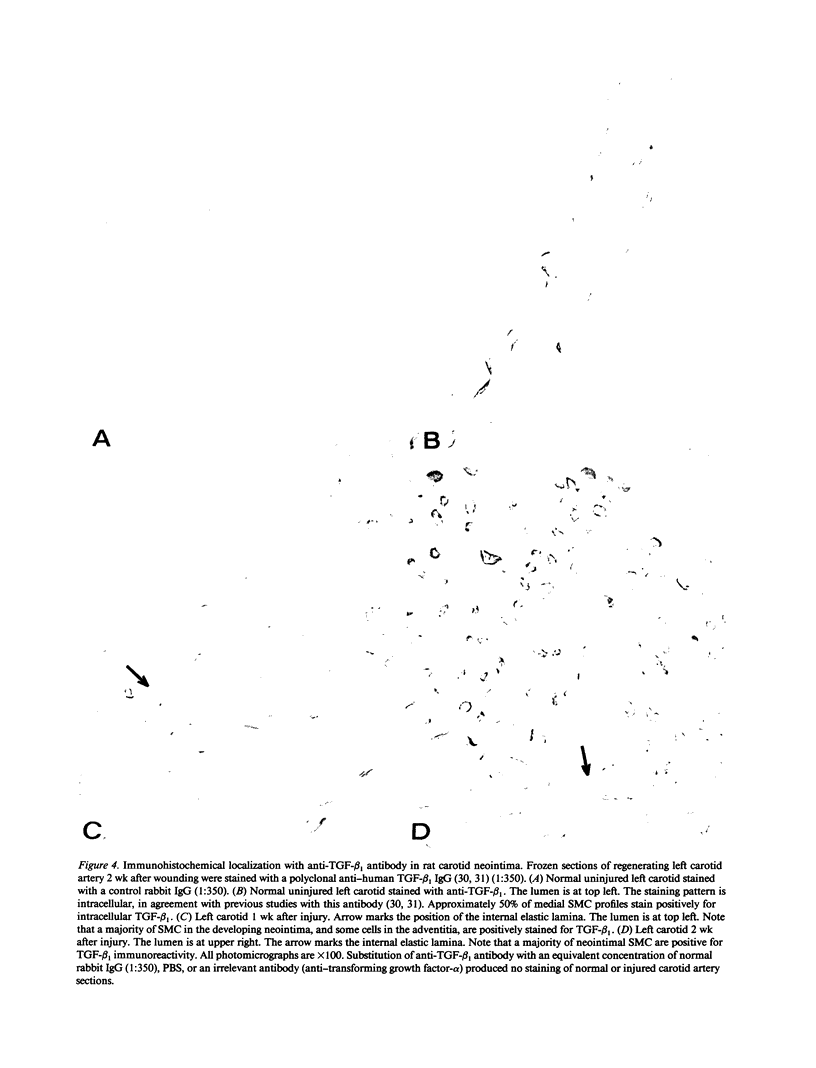
Repair of arterial injury produced by balloon angioplasty leads to the formation of a neointima and a narrowing of the vascular lumen. In this study, we examined the possibility that smooth muscle cells (SMC) in injured rat carotid arteries are stimulated to produce type-1 transforming growth factor-beta (TGF-beta 1) during neointima formation in vivo. Levels of TGF-beta 1 transcripts (2.4 kb) were significantly increased within 6 h after carotid injury and reached a maximum (five to sevenfold) by 24 h. Regenerating left carotids had sustained increases in TGF-beta 1 mRNA levels (about fivefold) over the next 2 wk, during which time a substantial neointimal thickening was formed. No changes in basal TGF-beta 1 mRNA levels were found in contralateral uninjured carotids at any of the times examined. Immunohistochemical studies showed that a large majority of neointimal SMC were stained for TGF-beta 1 protein in an intracellular pattern, consistent with active TGF-beta 1 synthesis in this tissue. Neointima formation and TGF-beta 1 immunoreactivity were correlated with increases in fibronectin, collagen alpha 2(I), and collagen alpha 1(III) gene expression. Infusion of purified, recombinant TGF-beta 1 into rats with a preexisting neointima produced a significant stimulation of carotid neointimal SMC DNA synthesis. These results suggest that TGF-beta 1 plays an important role as an endogenous growth regulatory factor produced by neointimal SMC themselves during progressive neointimal thickening after balloon angioplasty.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonelli-Orlidge A., Saunders K. B., Smith S. R., D'Amore P. A. An activated form of transforming growth factor beta is produced by cocultures of endothelial cells and pericytes. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4544–4548. doi: 10.1073/pnas.86.12.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assoian R. K., Sporn M. B. Type beta transforming growth factor in human platelets: release during platelet degranulation and action on vascular smooth muscle cells. J Cell Biol. 1986 Apr;102(4):1217–1223. doi: 10.1083/jcb.102.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett T. B., Benditt E. P. Platelet-derived growth factor gene expression in human atherosclerotic plaques and normal artery wall. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2810–2814. doi: 10.1073/pnas.85.8.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassols A., Massagué J. Transforming growth factor beta regulates the expression and structure of extracellular matrix chondroitin/dermatan sulfate proteoglycans. J Biol Chem. 1988 Feb 25;263(6):3039–3045. [PubMed] [Google Scholar]
- Battegay E. J., Raines E. W., Seifert R. A., Bowen-Pope D. F., Ross R. TGF-beta induces bimodal proliferation of connective tissue cells via complex control of an autocrine PDGF loop. Cell. 1990 Nov 2;63(3):515–524. doi: 10.1016/0092-8674(90)90448-n. [DOI] [PubMed] [Google Scholar]
- Bell L., Madri J. A. Effect of platelet factors on migration of cultured bovine aortic endothelial and smooth muscle cells. Circ Res. 1989 Oct;65(4):1057–1065. doi: 10.1161/01.res.65.4.1057. [DOI] [PubMed] [Google Scholar]
- Björkerud S., Bondjers G. Arterial repair and atherosclerosis after mechanical injury. 2. Tissue response after induction of a total local necrosis deep longitudinal injury. Atherosclerosis. 1971 Sep-Oct;14(2):259–276. doi: 10.1016/0021-9150(71)90055-4. [DOI] [PubMed] [Google Scholar]
- Braun L., Mead J. E., Panzica M., Mikumo R., Bell G. I., Fausto N. Transforming growth factor beta mRNA increases during liver regeneration: a possible paracrine mechanism of growth regulation. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1539–1543. doi: 10.1073/pnas.85.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. K., Hoshi H., McKeehan W. L. Transforming growth factor type beta specifically stimulates synthesis of proteoglycan in human adult arterial smooth muscle cells. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5287–5291. doi: 10.1073/pnas.84.15.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemmons D. R., Van Wyk J. J. Evidence for a functional role of endogenously produced somatomedinlike peptides in the regulation of DNA synthesis in cultured human fibroblasts and porcine smooth muscle cells. J Clin Invest. 1985 Jun;75(6):1914–1918. doi: 10.1172/JCI111906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clowes A. W., Clowes M. M., Au Y. P., Reidy M. A., Belin D. Smooth muscle cells express urokinase during mitogenesis and tissue-type plasminogen activator during migration in injured rat carotid artery. Circ Res. 1990 Jul;67(1):61–67. doi: 10.1161/01.res.67.1.61. [DOI] [PubMed] [Google Scholar]
- Clowes A. W., Clowes M. M., Reidy M. A. Kinetics of cellular proliferation after arterial injury. III. Endothelial and smooth muscle growth in chronically denuded vessels. Lab Invest. 1986 Mar;54(3):295–303. [PubMed] [Google Scholar]
- Clowes A. W., Reidy M. A., Clowes M. M. Kinetics of cellular proliferation after arterial injury. I. Smooth muscle growth in the absence of endothelium. Lab Invest. 1983 Sep;49(3):327–333. [PubMed] [Google Scholar]
- Derynck R., Lindquist P. B., Lee A., Wen D., Tamm J., Graycar J. L., Rhee L., Mason A. J., Miller D. A., Coffey R. J. A new type of transforming growth factor-beta, TGF-beta 3. EMBO J. 1988 Dec 1;7(12):3737–3743. doi: 10.1002/j.1460-2075.1988.tb03257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava R. A., McClure D. B. Fibronectin-associated transforming growth factor. J Cell Physiol. 1987 May;131(2):184–189. doi: 10.1002/jcp.1041310207. [DOI] [PubMed] [Google Scholar]
- Fingerle J., Au Y. P., Clowes A. W., Reidy M. A. Intimal lesion formation in rat carotid arteries after endothelial denudation in absence of medial injury. Arteriosclerosis. 1990 Nov-Dec;10(6):1082–1087. doi: 10.1161/01.atv.10.6.1082. [DOI] [PubMed] [Google Scholar]
- Fingerle J., Johnson R., Clowes A. W., Majesky M. W., Reidy M. A. Role of platelets in smooth muscle cell proliferation and migration after vascular injury in rat carotid artery. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8412–8416. doi: 10.1073/pnas.86.21.8412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanders K. C., Thompson N. L., Cissel D. S., Van Obberghen-Schilling E., Baker C. C., Kass M. E., Ellingsworth L. R., Roberts A. B., Sporn M. B. Transforming growth factor-beta 1: histochemical localization with antibodies to different epitopes. J Cell Biol. 1989 Feb;108(2):653–660. doi: 10.1083/jcb.108.2.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French J. E. Atherosclerosis in relation to the structure and function of the arterial intima, with special reference to th endothelium. Int Rev Exp Pathol. 1966;5:253–353. [PubMed] [Google Scholar]
- Gentry L. E., Webb N. R., Lim G. J., Brunner A. M., Ranchalis J. E., Twardzik D. R., Lioubin M. N., Marquardt H., Purchio A. F. Type 1 transforming growth factor beta: amplified expression and secretion of mature and precursor polypeptides in Chinese hamster ovary cells. Mol Cell Biol. 1987 Oct;7(10):3418–3427. doi: 10.1128/mcb.7.10.3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D., Ferrara N., Haaparanta T., Neufeld G. Basic fibroblast growth factor: expression in cultured bovine vascular smooth muscle cells. Eur J Cell Biol. 1988 Apr;46(1):144–151. [PubMed] [Google Scholar]
- Hansson G. K., Jonasson L., Holm J., Clowes M. M., Clowes A. W. Gamma-interferon regulates vascular smooth muscle proliferation and Ia antigen expression in vivo and in vitro. Circ Res. 1988 Oct;63(4):712–719. doi: 10.1161/01.res.63.4.712. [DOI] [PubMed] [Google Scholar]
- Huang S. S., O'Grady P., Huang J. S. Human transforming growth factor beta.alpha 2-macroglobulin complex is a latent form of transforming growth factor beta. J Biol Chem. 1988 Jan 25;263(3):1535–1541. [PubMed] [Google Scholar]
- Ignotz R. A., Massagué J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986 Mar 25;261(9):4337–4345. [PubMed] [Google Scholar]
- Kehrl J. H., Roberts A. B., Wakefield L. M., Jakowlew S., Sporn M. B., Fauci A. S. Transforming growth factor beta is an important immunomodulatory protein for human B lymphocytes. J Immunol. 1986 Dec 15;137(12):3855–3860. [PubMed] [Google Scholar]
- Keski-Oja J., Blasi F., Leof E. B., Moses H. L. Regulation of the synthesis and activity of urokinase plasminogen activator in A549 human lung carcinoma cells by transforming growth factor-beta. J Cell Biol. 1988 Feb;106(2):451–459. doi: 10.1083/jcb.106.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondaiah P., Van Obberghen-Schilling E., Ludwig R. L., Dhar R., Sporn M. B., Roberts A. B. cDNA cloning of porcine transforming growth factor-beta 1 mRNAs. Evidence for alternate splicing and polyadenylation. J Biol Chem. 1988 Dec 5;263(34):18313–18317. [PubMed] [Google Scholar]
- Liau G., Chan L. M. Regulation of extracellular matrix RNA levels in cultured smooth muscle cells. Relationship to cellular quiescence. J Biol Chem. 1989 Jun 15;264(17):10315–10320. [PubMed] [Google Scholar]
- Lindner V., Majack R. A., Reidy M. A. Basic fibroblast growth factor stimulates endothelial regrowth and proliferation in denuded arteries. J Clin Invest. 1990 Jun;85(6):2004–2008. doi: 10.1172/JCI114665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons R. M., Gentry L. E., Purchio A. F., Moses H. L. Mechanism of activation of latent recombinant transforming growth factor beta 1 by plasmin. J Cell Biol. 1990 Apr;110(4):1361–1367. doi: 10.1083/jcb.110.4.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madri J. A., Reidy M. A., Kocher O., Bell L. Endothelial cell behavior after denudation injury is modulated by transforming growth factor-beta1 and fibronectin. Lab Invest. 1989 Jun;60(6):755–765. [PubMed] [Google Scholar]
- Majack R. A. Beta-type transforming growth factor specifies organizational behavior in vascular smooth muscle cell cultures. J Cell Biol. 1987 Jul;105(1):465–471. doi: 10.1083/jcb.105.1.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majack R. A., Majesky M. W., Goodman L. V. Role of PDGF-A expression in the control of vascular smooth muscle cell growth by transforming growth factor-beta. J Cell Biol. 1990 Jul;111(1):239–247. doi: 10.1083/jcb.111.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majesky M. W., Benditt E. P., Schwartz S. M. Expression and developmental control of platelet-derived growth factor A-chain and B-chain/Sis genes in rat aortic smooth muscle cells. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1524–1528. doi: 10.1073/pnas.85.5.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majesky M. W., Daemen M. J., Schwartz S. M. Alpha 1-adrenergic stimulation of platelet-derived growth factor A-chain gene expression in rat aorta. J Biol Chem. 1990 Jan 15;265(2):1082–1088. [PubMed] [Google Scholar]
- Majesky M. W., Reidy M. A., Bowen-Pope D. F., Hart C. E., Wilcox J. N., Schwartz S. M. PDGF ligand and receptor gene expression during repair of arterial injury. J Cell Biol. 1990 Nov;111(5 Pt 1):2149–2158. doi: 10.1083/jcb.111.5.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majesky M. W., Schwartz S. M., Clowes M. M., Clowes A. W. Heparin regulates smooth muscle S phase entry in the injured rat carotid artery. Circ Res. 1987 Aug;61(2):296–300. doi: 10.1161/01.res.61.2.296. [DOI] [PubMed] [Google Scholar]
- Miller D. A., Lee A., Matsui Y., Chen E. Y., Moses H. L., Derynck R. Complementary DNA cloning of the murine transforming growth factor-beta 3 (TGF beta 3) precursor and the comparative expression of TGF beta 3 and TGF beta 1 messenger RNA in murine embryos and adult tissues. Mol Endocrinol. 1989 Dec;3(12):1926–1934. doi: 10.1210/mend-3-12-1926. [DOI] [PubMed] [Google Scholar]
- Miller D. A., Lee A., Pelton R. W., Chen E. Y., Moses H. L., Derynck R. Murine transforming growth factor-beta 2 cDNA sequence and expression in adult tissues and embryos. Mol Endocrinol. 1989 Jul;3(7):1108–1114. doi: 10.1210/mend-3-7-1108. [DOI] [PubMed] [Google Scholar]
- Miyazono K., Hellman U., Wernstedt C., Heldin C. H. Latent high molecular weight complex of transforming growth factor beta 1. Purification from human platelets and structural characterization. J Biol Chem. 1988 May 5;263(13):6407–6415. [PubMed] [Google Scholar]
- Owens G. K., Geisterfer A. A., Yang Y. W., Komoriya A. Transforming growth factor-beta-induced growth inhibition and cellular hypertrophy in cultured vascular smooth muscle cells. J Cell Biol. 1988 Aug;107(2):771–780. doi: 10.1083/jcb.107.2.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penttinen R. P., Kobayashi S., Bornstein P. Transforming growth factor beta increases mRNA for matrix proteins both in the presence and in the absence of changes in mRNA stability. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1105–1108. doi: 10.1073/pnas.85.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappolee D. A., Mark D., Banda M. J., Werb Z. Wound macrophages express TGF-alpha and other growth factors in vivo: analysis by mRNA phenotyping. Science. 1988 Aug 5;241(4866):708–712. doi: 10.1126/science.3041594. [DOI] [PubMed] [Google Scholar]
- Reidy M. A. A reassessment of endothelial injury and arterial lesion formation. Lab Invest. 1985 Nov;53(5):513–520. [PubMed] [Google Scholar]
- Sarzani R., Brecher P., Chobanian A. V. Growth factor expression in aorta of normotensive and hypertensive rats. J Clin Invest. 1989 Apr;83(4):1404–1408. doi: 10.1172/JCI114029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y., Rifkin D. B. Inhibition of endothelial cell movement by pericytes and smooth muscle cells: activation of a latent transforming growth factor-beta 1-like molecule by plasmin during co-culture. J Cell Biol. 1989 Jul;109(1):309–315. doi: 10.1083/jcb.109.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S. M., Campbell G. R., Campbell J. H. Replication of smooth muscle cells in vascular disease. Circ Res. 1986 Apr;58(4):427–444. doi: 10.1161/01.res.58.4.427. [DOI] [PubMed] [Google Scholar]
- Schwartz S. M., Stemerman M. B., Benditt E. P. The aortic intima. II. Repair of the aortic lining after mechanical denudation. Am J Pathol. 1975 Oct;81(1):15–42. [PMC free article] [PubMed] [Google Scholar]
- Schwarzbauer J. E., Tamkun J. W., Lemischka I. R., Hynes R. O. Three different fibronectin mRNAs arise by alternative splicing within the coding region. Cell. 1983 Dec;35(2 Pt 1):421–431. doi: 10.1016/0092-8674(83)90175-7. [DOI] [PubMed] [Google Scholar]
- Seifert R. A., Schwartz S. M., Bowen-Pope D. F. Developmentally regulated production of platelet-derived growth factor-like molecules. Nature. 1984 Oct 18;311(5987):669–671. doi: 10.1038/311669a0. [DOI] [PubMed] [Google Scholar]
- Shimokado K., Raines E. W., Madtes D. K., Barrett T. B., Benditt E. P., Ross R. A significant part of macrophage-derived growth factor consists of at least two forms of PDGF. Cell. 1985 Nov;43(1):277–286. doi: 10.1016/0092-8674(85)90033-9. [DOI] [PubMed] [Google Scholar]
- Sjölund M., Hedin U., Sejersen T., Heldin C. H., Thyberg J. Arterial smooth muscle cells express platelet-derived growth factor (PDGF) A chain mRNA, secrete a PDGF-like mitogen, and bind exogenous PDGF in a phenotype- and growth state-dependent manner. J Cell Biol. 1988 Feb;106(2):403–413. doi: 10.1083/jcb.106.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow A. D., Bolender R. P., Wight T. N., Clowes A. W. Heparin modulates the composition of the extracellular matrix domain surrounding arterial smooth muscle cells. Am J Pathol. 1990 Aug;137(2):313–330. [PMC free article] [PubMed] [Google Scholar]
- Spaet T. H., Stemerman M. B., Veith F. J., Lejnieks I. Intimal injury and regrowth in the rabbit aorta; medial smooth muscle cells as a source of neointima. Circ Res. 1975 Jan;36(1):58–70. doi: 10.1161/01.res.36.1.58. [DOI] [PubMed] [Google Scholar]
- Stemerman M. B., Ross R. Experimental arteriosclerosis. I. Fibrous plaque formation in primates, an electron microscope study. J Exp Med. 1972 Oct 1;136(4):769–789. doi: 10.1084/jem.136.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stimac E., Groppi V. E., Jr, Coffino P. Inhibition of protein synthesis stabilizes histone mRNA. Mol Cell Biol. 1984 Oct;4(10):2082–2090. doi: 10.1128/mcb.4.10.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson N. L., Flanders K. C., Smith J. M., Ellingsworth L. R., Roberts A. B., Sporn M. B. Expression of transforming growth factor-beta 1 in specific cells and tissues of adult and neonatal mice. J Cell Biol. 1989 Feb;108(2):661–669. doi: 10.1083/jcb.108.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tso J. Y., Sun X. H., Kao T. H., Reece K. S., Wu R. Isolation and characterization of rat and human glyceraldehyde-3-phosphate dehydrogenase cDNAs: genomic complexity and molecular evolution of the gene. Nucleic Acids Res. 1985 Apr 11;13(7):2485–2502. doi: 10.1093/nar/13.7.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakefield L. M., Smith D. M., Flanders K. C., Sporn M. B. Latent transforming growth factor-beta from human platelets. A high molecular weight complex containing precursor sequences. J Biol Chem. 1988 Jun 5;263(16):7646–7654. [PubMed] [Google Scholar]
- Walker L. N., Bowen-Pope D. F., Ross R., Reidy M. A. Production of platelet-derived growth factor-like molecules by cultured arterial smooth muscle cells accompanies proliferation after arterial injury. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7311–7315. doi: 10.1073/pnas.83.19.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox J. N., Smith K. M., Williams L. T., Schwartz S. M., Gordon D. Platelet-derived growth factor mRNA detection in human atherosclerotic plaques by in situ hybridization. J Clin Invest. 1988 Sep;82(3):1134–1143. doi: 10.1172/JCI113671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkles J. A., Friesel R., Burgess W. H., Howk R., Mehlman T., Weinstein R., Maciag T. Human vascular smooth muscle cells both express and respond to heparin-binding growth factor I (endothelial cell growth factor). Proc Natl Acad Sci U S A. 1987 Oct;84(20):7124–7128. doi: 10.1073/pnas.84.20.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood L., Theriault N., Vogeli G. Complete nucleotide sequence of the N-terminal domains of the murine alpha-1 type-III collagen chain. Gene. 1987;61(2):225–230. doi: 10.1016/0378-1119(87)90117-x. [DOI] [PubMed] [Google Scholar]