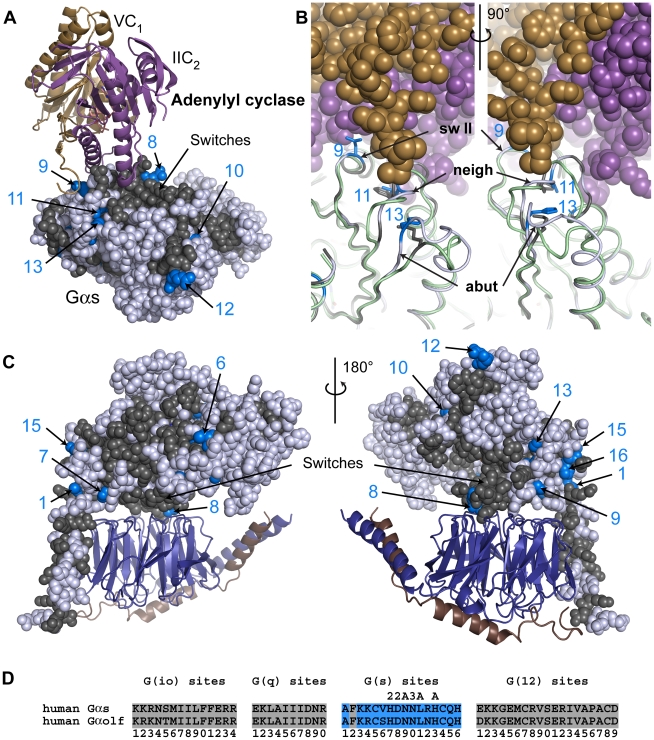
Figure 6. G(s) class-distinctive sites in structural context.
(A) The structure of the catalytic domains of adenylyl cyclase (VC1 in sand cartoon, IIC2 in purple cartoon) bound to an activated Gαs (Gαs •GTPγS) (PDB ID 1AZS). In all structural panels in this figure, Gαs is shown as spheres or cartoon with core residues colored gray if they are conserved between Gα subunits (either INV (invariant) or η (non-distinct) amino acids) while G(s)-distinctive sites are colored blue only if they contain a ∂ amino acid. Non-core residues and d sites are colored white. G(s)-distinctive sites are numbered according to their position in the signature sequence (see panel (D)). (B) Superimposition of Gαs (light gray cartoon with ∂ amino acids at G(s) sites of interest rendered as blue sticks) and Gαi1 (PDB ID 1GIA in light green cartoon with corresponding η amino acids at G(s) sites in sticks and colored gray) highlighting sequence and backbone conformational changes in switch II (“sw II”) and loops near the adenylyl cyclase interface. The two views are related by a 90° rotation about the vertical axis. VC1 is in sand spheres and IIC2 is in purple spheres. G(s) site 11 lies in a loop neighboring switch II that forms part of the binding interface (“neigh”) while G(s) site 13 is in a loop that abuts the binding interface (“abut”). (C) Homology model of Gαs •GDP (sphere display) bound to Gβ•Gγ (deepblue/copper cartoon) heterodimer. Two orientations related by a 180° rotation about the vertical axis are shown. (D) Signature sequences are formed by grouping all distinctive sites for a given class together, removing all residues between individual distinctive sites of the noted class. The distinctive sites for each class are presented in order from the N-terminus to the C-terminus and numbered accordingly. Amino acids that correspond to the ∂ values at the G(s) site are colored blue. Sites that have been proposed to be important to the interaction with adenylyl cyclase are denoted by ‘A’ above the site, while additional sites in switches II or III are denoted by ‘2’ or ‘3’, respectively, above the site.