Abstract
Objectives
Patient activation can improve health outcomes for chronic diseases that disproportionately affect the elderly. The present study evaluated the impact of an activation intervention delivered in community senior centers.
Participants
One hundred and sixteen senior participants.
Setting
Two Los Angeles community senior centers.
Intervention
Participants were invited to attend group screenings of video programs intended to inform and motivate self-management of chronic conditions common among seniors. Screenings were followed by moderated discussions reinforcing active patient participation in chronic disease management. Screenings were scheduled over the course of 12 weeks.
Design and Measures
One center was assigned by coin-toss to an encouragement condition, in which participants received a $50 gift card if they attended at least 3 group screenings. Participants in the non-encouraged center received no incentive for attendance. Validated study measures for patient activation, physical activity and health-related quality of life were completed at baseline, 12 weeks and 6 months following enrollment.
Results
Participants attending the encouraged senior center were more likely to attend 3 or more group screenings (77.8% vs. 47.2%, p=.001). At 6-month follow-up, participants from either center who attended 3 or more group screenings (n=74, 64%) reported significantly greater activation (p=.000), more minutes walking (p=.000) and engaging in vigorous physical activity (p=.006) and better health-related quality of life (SF-12 MCS: p=.000; SF-12 PCS: p=.002).
Conclusion
Delivering this pilot intervention in community senior centers is a potentially promising approach to activate seniors that warrants further investigation for improving chronic disease outcomes.
Keywords: Chronic diseases, patient activation, self-management
INTRODUCTION
Older adults are disproportionately affected by chronic diseases, including coronary artery disease, congestive heart failure, diabetes and chronic low back pain1. Cardiovascular disease is the leading cause of death in the United States, accounting for 29% of all deaths in 20012. Diabetes is the sixth leading cause of death and when managed sub-optimally causes complications that significantly impair quality of life, in addition to contributing to vascular disease2. Chronic low back pain affects as many as 50% of community-dwelling adults, causing substantial impairments in functioning and health-related quality of life3. Among adults 65 and older, as many as 85% suffer from one or more chronic diseases1. A recent estimate suggests that individuals with one or more chronic conditions account for almost 50% of total health care expenditures4.
Patient activation – defined as being able to self-manage symptoms and problems, engaging in activities that maintain functioning and reduce health declines, and being involved in clinical decision-making – can significantly improve health outcomes in chronic disease management5-8. Nevertheless, the question of how to best activate patients remains unanswered6,9. The Chronic Care Model has been proposed as a template for enabling primary care to respond effectively to the challenges of managing patients with chronic conditions10. However, it has been argued that the full implementation of this model has been limited by the lack of effective strategies to activate patients9. The urgency of answering the question of how to activate patients is strengthened by evidence that patient activation is mutable and can be increased among older adults with chronic conditions7.
The vast majority of patients’ self-management activities for chronic conditions happen outside of health care settings in the community, for example reducing dietary fat, increasing physical activity or self-monitoring blood glucose and pressure. For this reason, we sought to conduct an initial test of a novel but relatively simple intervention for seniors with chronic conditions, to determine whether it could increase their level of activation. Participants were recruited from 2 multipurpose community senior centers. A recent estimate suggests that over 10 million seniors are served annually by 14,000 centers throughout the United States11.
METHODS
Setting
A large number of older adults regularly use community senior centers to access services and seek social support12. These centers provide access to health and wellness services and often include small fitness centers. They also organize social activities for seniors, such as group card games, dances and field trips to other locations. Many centers also provide access to hot meals, which for some seniors are federally subsidized. Findings from several studies suggest that senior centers can serve as an effective venue for providing health education and changing the health behavior of older adults13-15. The present study took place in two community senior centers in greater Los Angeles. One senior center was located in a low-income predominately African American neighborhood and serves approximately 8,000 seniors according to the center director (Center 1). The other center was located in a middle income ethnically mixed neighborhood and serves approximately 5,000 seniors (Center 2). The senior centers were selected because they previously participated in and were receptive to health promotion research conducted by UCLA investigators. These 2 previous intervention studies took place from 2003-2006 and in 2007, respectively.
Intervention
Each of the 2 senior centers was provided a set of 5 video programs (each between 20-45 minutes long) developed by the Foundation for Informed Medical Decision Making. Four of the 5 video programs were about chronic diseases common among older adults, including coronary artery disease, congestive heart failure, type II diabetes and chronic low back pain. The fifth program was focused on the role of advance directives for articulating health care preferences in the case of incapacitation or at the end of life. Each of the chronic disease programs emphasized the importance of engaging in self-care behaviors known to improve management of the respective disease, such as reducing dietary fat and increasing physical activity for coronary artery disease, managing sodium intake and monitoring body weight for congestive heart failure, monitoring hemoglobin A1c, blood pressure and cholesterol for diabetes, and engaging in physical activity to manage low back pain. The programs combined education with a motivational tone, using interviews with real patients to illustrate different individuals’ ways of increasing self-management of chronic conditions. During the 12 week intervention period, in both community senior centers, each program was shown in group screenings on multiple occasions on different days and at different times to maximize the opportunities for seniors to attend. The group screenings were led by a single trained facilitator in both centers who moderated discussion with the participants after viewing the video program. The facilitator, who was a member of the research team, had a Bachelor's degree and received training from the investigators in the basic principles of motivational interviewing16. During the discussions, the facilitator continued to reinforce the importance of active self-management to improve chronic disease outcomes. Attendance at group screenings was captured with sign-in sheets in both senior centers.
Design
Our primary hypothesis was that repeated exposure to the message that active self-management would improve chronic disease outcomes would lead to greater patient activation, regardless of the specific chronic disease context. We considered different potential research designs to increase exposure to the intervention materials and test this hypothesis. We ruled out assigning one community senior center to the intervention and the other to a no intervention control condition because receiving no intervention would likely lead to differential completion rates of study measures. Similarly, we also ruled out randomization at the individual level, as this would entail a high risk of contamination within each senior center. Instead we decided to assign one center by coin-toss to an encouragement condition, in which participants would receive a $50 gift card if they attended three or more different intervention group screenings. Study procedures were identical in the non-encouraged center, except that participants did not receive the $50 incentive for attending 3 or more group screenings. Participants in both centers were encouraged to attend group screenings. The study protocol was reviewed and approved by the UCLA Institutional Review Board (Clinicaltrials.gov identifier: NCT00651495).
Participant recruitment
Seniors attending both centers were invited to information sessions that provided simple refreshments and described the intervention program. The information sessions were conducted on multiple occasions in equal numbers in both centers. The sessions described the availability of the video programs focused on living with chronic conditions and informed seniors that they could view these programs on their own (with equipment provided to the senior centers for this purpose) or participate in group screenings. Seniors in both centers were encouraged to attend group screenings to take advantage of the opportunity to discuss the program content with peers and the trained facilitator. Watching the programs individually or participating in the group screening did not require participation in the study testing our hypothesis. At the end of the information session, seniors were told that volunteers were sought to help evaluate the intervention program. Volunteers had to meet the following 4 criteria: (1) age 55 or older, (2) able to ambulate on their own, (3) able to complete questionnaires without assistance, and (4) able to read and write English. Individuals attending the community senior center assigned to the encouragement condition who agreed to participate in the evaluation were told that they could earn a $50 gift card for attending three or more group screenings (the incentive was not available to individuals who did not participate in the evaluation of the intervention). Individuals who were willing to participate in the evaluation of the intervention reviewed and completed an informed consent document and completed the baseline questionnaire. The 12-week intervention period began after enrollment into the study was completed. Our target sample size was 60 seniors per center (N=120).
Measures
Participants completed study measures at baseline, following the 12 week intervention period and 6 months after enrolling in the study. All participants received a $10 gift card for each completed survey for a total of $30 for completing all 3 surveys. These payments were in addition to the $50 participants attending the center assigned to the encouragement condition could earn for attending 3 group screenings. We achieved completion rates of 98.3% for both follow-up surveys. Follow-up completion was similar across both community senior centers. With the exception of group screening attendance, which was recorded with sign-in sheets at each screening, all study measures were based on self-report. Participants answered demographic and health history questions, including history of chronic diseases and number of prescribed medications, at baseline. At each assessment point, participants completed the previously validated Patient Activation Measure (PAM)5,17, a brief previously validated measure of physical activity18 and the SF-12 measure of health related quality of life 19. The PAM is a 13-item measure that assesses patients’ self-rated ability to take preventive actions, manage symptoms of medical problems, find and use appropriate medical care and work with their health care providers to make decisions about their care17. The PAM produces a single score that has been shown to be reliable and valid. Higher scores indicate greater activation and correlate with better chronic disease self-management and greater engagement in preventive behaviors6,9. Our physical activity measure consisted of 9-items and has been shown to have comparable sensitivity and validity to the widely used 7-day Physical Activity Recall18. The measure enables estimation of the number of minutes an individual engaged in walking, moderate and vigorous physical activity in the past week. The SF-12 is a shortened version of the widely used SF-36 measure of health-related quality of life19. We opted for the SF-12 in order to reduce respondent burden and because the measure has been shown to have good validity despite using fewer items than the SF-3619. In addition to these measures, participants also answered questions assessing subjective perceptions of change in the following domains at 12 weeks and 6 months: (1) willingness to ask questions of a physician, (2) confidence in one's ability to ask questions of a physician, (3) general health, (4) who is responsible for managing one's health, and (5) what one does to manage one's health20. Each of these questions had 7-point Likert-type response options with a mid-point of no change and anchors indicating positive or negative change. The questions were internally consistent with a Cronbach's alpha of .92. At follow-up, participants also answered several open-ended questions that asked about any changes they had made in how they treat their condition as a result of participating in the intervention program.
Statistical analysis
Continuous measures were analyzed with Analysis of Variance models (ANOVA). Baseline scores were included in the models as a covariate, as were the number of chronic diseases reported. We tested the effect of 2 independent variables. First we tested whether outcomes differed by senior center attended. Second, because our primary interest was in the effect of repeated participation in group screenings on our outcome measures, we dichotomized participants based on whether they attended 3 or more or 2 or fewer chronic disease program group screenings. We also tested for interaction effects of these two variables to determine whether there were differential effects of repeated exposure to the programs depending on which senior center participants were recruited from. We report estimated marginal means for each ANOVA model, which adjusts the means taking into account the covariates included in the model. Categorical measures were analyzed with Pearson Chi-Square. Data were analyzed with SPSS 17.0.
RESULTS
We enrolled 116 participants into the study between April 2, 2008 and May 15, 2008. Before evaluating our main study hypotheses we compared the baseline characteristics of participants by community senior center and whether or not they attended 3 or more group screening sessions (see Table 1). Participants recruited from the community senior center assigned to the encouragement condition (Center 1) were somewhat younger, more likely to be African American, had fewer years of education and reported lower household incomes. These differences in demographic characteristics reflect the neighborhood characteristics of the respective geographical locations of the community senior centers. They also reported significantly more minutes walking per week and higher SF-12 mental health component scores.
Table 1.
Baseline participant characteristics
| Center 1* (n=63) | Center 2 (n=53) | Prob. | 2 or fewer screenings (n=42) | 3 or more screenings (n=74) | Prob. | |
|---|---|---|---|---|---|---|
| Age Mean (±SD) | 70.61 (7.71) | 73.65 (8.34) | .046 | 73.95 (9.27) | 70.86 (7.18) | .049 |
| Female | 83.9% | 71.7% | .115 | 61.9% | 87.7% | .001 |
| Widowed | 44.1% | 32.0% | .157 | 36.6% | 39.7% | .159 |
| Ethnicity | ||||||
| African Amer. | 93.7% | 19.6% | 43.9% | 69.9% | ||
| Caucasian | 0.0% | 58.8% | .000 | 41.5% | 17.8% | .053 |
| Latino | 4.8% | 7.8% | 7.3% | 5.5% | ||
| Education (More than High School) | 50.0% | 92.5% | .000 | 78.0% | 64.9% | .269 |
| Income ($35k or less) | 63.6% | 46.0% | .004 | 48.7% | 59.1% | .052 |
| Number of chronic conditions Mean (±SD) | 2.05 (0.96) | 1.81 (1.14) | .223 | 1.90 (1.01) | 1.96 (1.07) | .796 |
| Number of Rx medications Mean (±SD) | 3.98 (3.19) | 3.35 (2.64) | .267 | 3.72 (2.29) | 3.67 (3.27) | .937 |
| Baseline PAM Score Mean (±SD) | 62.46 (14.35) | 66.17 (16.04) | .192 | 63.64 (13.59) | 64.45 (16.11) | .783 |
| Baseline Walking - min/week Mean (±SD) | 152.24 (137.46) | 102.19 (100.49) | .038 | 101.74 (94.82) | 143.89 (135.16) | .098 |
| Baseline Moderate Activity - min/week (Mean, (±SD)) | 81.81 (87.43) | 72.24 (90.01) | .579 | 72.18 (76.48) | 80.44 (94.87) | .644 |
| Baseline Vigorous Activity - min/week (Mean, (±SD)) | 82.59 (90.43) | 57.23 (80.72) | .140 | 52.21 (57.33) | 80.29 (94.88) | .122 |
| Baseline SF 12 – Physical Component Score (Mean, (±SD)) | 45.25 (6.62) | 47.75 (7.08) | .056 | 46.47 (7.07) | 46.41 (6.90) | .968 |
| Baseline SF 12 – Mental Health Component Score (Mean, (±SD)) | 55.80 (4.97) | 52.64 (6.60) | .005 | 54.03 (6.23) | 54.45 (5.89) | .724 |
Randomized to the encouragement condition
The financial incentive was successful in increasing participation in three or more group screenings. In Center 1 77.8% of participants attended 3 or more group screenings, compared with 47.2% of participants in Center 2 (p=.001). Correspondingly, as also shown in Table 1, there were some differences in baseline characteristics comparing those who attended 3 or more group screenings with those attending fewer. These participants were somewhat younger, more likely to be female and were marginally more likely to be African American and have lower household incomes. Although the differences were non-significant, participants who attended 3 or more group screenings reported somewhat more physical activity at baseline. However, number of self-reported chronic conditions, prescription medications and baseline PAM scores were virtually identical comparing participants who attended 3 or more group screenings versus those who did not. Interestingly, although both community senior centers were provided with a television and DVD player, to enable seniors to watch the programs on their own, no seniors in either community center watched the programs outside of the scheduled group screenings.
Primary outcome measures
Controlling for baseline scores and number of chronic diseases, differences in PAM scores at 12 weeks comparing participants from the 2 centers were non-significant (p=.321). However, participants who attended 3 or more chronic disease group screenings in either center reported significantly higher PAM scores at 12 weeks (Mean = 66.16 (SE=1.34) vs. 61.58 (SE=1.74); p=.039). The interaction between center and number of screenings attended was non-significant. At 6-month follow-up there were significant differences in PAM scores comparing the 2 centers (p=.006) and comparing those who attended 3 or more group screenings to those who attended fewer or no group screenings (Mean = 76.79 (SE=1.45) vs. 61.36 (SE=1.88); p=.000). The interaction between center and number of screenings attended was also significant (p=.043), with greater differences by group screening attendance in center 1 compared to center 2. Table 2 shows the proportion of participants who increased their activation and the corresponding average increase at 6-month follow-up by baseline activation level and number of screenings attended. A significantly greater proportion of participants who attended 3 or more screenings increased their activation level (p=.000).
Table 2.
Proportion of participants with increases in activation at 6-month follow-up by number of screenings attended and baseline activation level
| Participants attending 2 or fewer screenings (n=42) | Participants attending 3 or more screenings (n=72) | |||||
|---|---|---|---|---|---|---|
| Baseline Activation Level* | n | % increasing activation | Mean (±SD) increase in activation | n | % increasing activation | Mean (±SD) increase in activation |
| Level 1 | 3 | 100% | 15.4 (6.9) | 7 | 100% | 32.2 (6.8) |
| Level 2 | 8 | 75% | 12.4 (16.0) | 17 | 100% | 27.4 (7.3) |
| Level 3 | 18 | 56% | 1.1 (17.1) | 21 | 100% | 17.5 (6.5) |
| Level 4 | 13 | 15% | -13.8 (15.4) | 27 | 33% | -6.7 (14.1) |
Patient Activation scores range from 0-100 and are divided into 4 levels. Level 1 (scores 47.0 or lower) is associated with not believing that one has a role to play in self-management of chronic conditions. Level 2 (scores 47.1-55.1) is associated with a lack of knowledge and confidence to take action in self-management of chronic conditions. Level 3 (scores 55.2-67.0) is associated with beginning to take action in self-management, and Level 4 (scores greater than 67.1) is associated with maintaining behavior change, though individuals may still experience difficulties overcoming obstacles9.
Controlling for baseline physical activity and number of chronic diseases, participants who attended 3 or more chronic disease group screenings reported significantly more minutes per week walking at 12 weeks (Mean = 150.69 (SE=12.71) vs. 78.79 (SE=17.42); p=.001) and 6-month follow-up (Mean = 102.30 (SE=6.19) vs. 55.31 (SE=8.48); p=.000) than those who attended fewer or no group screenings. Differences by center attended were non-significant and there was no interaction effect. Differences in minutes of vigorous physical activity per week were non-significant at 12-weeks by center (p=.98) and screenings attended (p=.17). However, at 6-months there were significant differences in vigorous physical activity by center (p=.015) and number of screenings attended (Mean = 54.29 (SE=6.30) vs. 23.79 (SE=8.80); p=.006). The interaction was non-significant. Differences in moderate physical activity were non-significant at both time points by center and number of screenings attended. The data indicated that participants did not substitute one type of physical activity for another (results not shown).
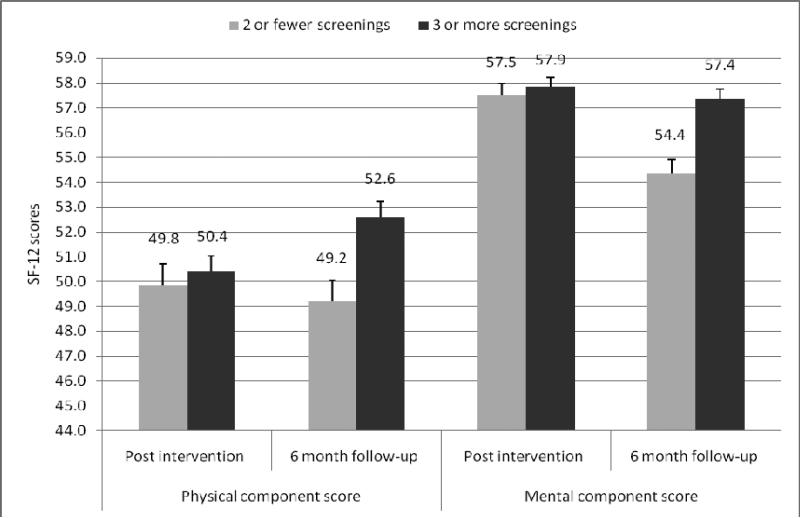
There were no differences in SF-12 scores at 12 weeks. However, at 6-month follow-up, participants who attended 3 or more group screenings reported significantly higher physical (p=.002) and mental health component scores (p=.000). Differences by center attended were non-significant and there was no interaction effect, for both physical and mental component scores. Figure 1 shows differences in SF-12 physical component and mental health component scores comparing those who attended 3 or more group screenings versus those who did not.
Figure 1.
Health-related quality of life by number of screenings attended
Subjective perceptions of change
Table 3 shows participants’ subjective perceptions of change at 6-month follow-up by number of group screenings attended. Participants who attended 3 or more group screenings indicated significantly greater change in their willingness (p=.000) and confidence to ask questions of their physician (p=.000). They also reported greater change in their sense of personal responsibility for their health (p=.000) and making more changes in activities to manage their health (p=.000). Finally, they perceived significantly greater change in their subjective health rating (p=.000). The differences were less pronounced comparing participants from the 2 centers, but were also significant with the exception of willingness to ask questions of a physician (results not shown).
Table 3.
Participants’ subjective perceptions of change at 6-month follow-up by number of screenings attended
| How willing are you now to ask your doctor questions about your treatment? | 3 or more screenings | No change | A little more willing | Somewhat more willing | Much more willing | Prob. |
| 1.4% | 13.9% | 68.1% | 16.7% | .000 | ||
| 2 or fewer screenings | 35.7% | 23.8% | 35.7% | 4.8% | ||
| How confident are you now that you can ask your doctor questions about your treatment? | 3 or more screenings | No change | A little more confident | Somewhat more confident | Much more confident | .000 |
| 2.8% | 6.9% | 62.5% | 27.8% | |||
| 2 or fewer screenings | 38.1% | 31.0% | 28.6% | 2.4% | ||
| Have you experienced any changes in how you now think about who is responsible for managing your health? | 3 or more screenings | No change | A little more my responsibility | Somewhat more my responsibility | Much more my responsibility | .000 |
| 4.2% | 5.6% | 44.4% | 45.8% | |||
| 2 or fewer screenings | 40.5% | 16.7% | 28.6% | 14.3% | ||
| Have you made any changes in what you now do to manage your health? | 3 or more screenings | No change | Doing a little more to manage my health | Doing somewhat more to manage my health | Doing much more to manage my health | .000 |
| 5.6% | 16.7% | 72.2% | 5.6% | |||
| 2 or fewer screenings | 42.9% | 40.5% | 16.7% | 0.0% | ||
| In general, how would you rate your health now, as a result of what you learned in this program? | 3 or more screenings | No change | A little better | Somewhat better | Much Better | .000 |
| 13.9% | 31.9% | 43.1% | 11.1% | |||
| 2 or fewer screenings | 61.9% | 26.2% | 11.9% | 0.0% |
Completion of advance directive
Our 6-month follow-up survey queried whether participants had completed an advance directive or intended to complete one since beginning participation in the study. Participants who attended the group screening focused on advance directives (58.6% of participants across both centers) were significantly more likely to report having recently completed an advance directive (13.4% versus 2.1% of those who did not attend; p=.036) or having an intention to complete one (41.0% versus 17.4% of those who did not attend; p=.009). Overall, participants who attended the group screening focused on advance directives were 4 times as likely as participants who did not attend the screening to have completed or intend to complete an advance directive (OR = 4.03, 95% CI = 1.62-10.05, p=.003).
Corroborating qualitative data
At 6-month follow-up participants answered several open-ended questions querying whether they had spoken to their physician about something they learned from the intervention video programs, whether they had decided with their physician to change their treatment regimen, or whether they had changed how they treated their chronic condition. Table 4 shows a sample of open-ended survey responses and the participants’ corresponding changes in activation from baseline to 6-month follow-up. These responses corroborate our quantitative findings, by illustrating specific ways in which participants became activated as a result of participating in our intervention. Participants described asking about specific interventions they learned about (e.g., statin drugs to lower cholesterol), making decisions with their physicians to alter their treatment regimens, and engaging in more self-care behaviors.
Table 4.
Sample participant responses to open ended survey questions
| Open-ended survey question | |||
|---|---|---|---|
| Participant demographics and increase in activation (Level & absolute PAM scores) from baseline to 6-month follow-up | Did you talk to your doctor about something you learned in a video or discussion about a video? | Did you and your doctor decide to make changes to your treatment? | As a result of something you learned, did you make any changes in what you do to treat your condition? |
|
65 year-old African American female Baseline Activation Level: 4 6-month Follow-Up Level: 4 PAM Score: +23.1 points |
I was not on a Statin and after watching the video I decided I wanted to take one to help lower my cholesterol. Also, I changed my PCP because I felt that my old PCP was too arrogant and didn't allow me to ask questions or give my input. So I told him that I did have every right to find a doctor that cares about my needs. | I asked about Statins and my new PCP agreed and I am now taking a Statin. | I took a class for Health Living at Kaiser. |
|
65-year-old African American female Baseline Activation Level: 2 6-month Follow-Up Level: 4 PAM Score: +22.4 points |
Yes, I talked to my doctor about changing my medication for my heart condition because it was making me feel really tired. I found out that my kidneys were not getting enough blood. Now that I changed the medication, I feel so much better. | I felt more informed and that I knew a little more about side effects to my medications. So I felt like I could have more input in my treatment plan. | Yes, I now ask more questions. I feel like I do have an important role in my care. |
|
64-year-old African American female Baseline Activation Level: 2 6-month Follow-Up Level: 4 PAM Score: +35.4 points |
We talked about my cholesterol meds and how they were making me feel. He switched me to another kind and now I don't feel as nauseous as I used to. | I asked my doctor that I wanted him to test me for diabetes. So next week I have a fasting test to take a look at my sugar levels. I feel much more empowered now because I learned so many things that now I write down questions before I see my doctor so that I don't forget. | Yes, I am walking more and testing my blood pressure at least once a day. |
|
68-year old African American female Baseline Activation Level: 3 6-month Follow-Up Level: 4 PAM Score: +23.1 points |
I mentioned to my doctor that I had seen a video on diabetes and that it really motivated me to exercise more. I asked him what exercises he could recommend for me. Also, I asked him about Statins and if I was taking one. | Yes, I found out I was taking a Statin already, but my doctor switched me to another med because I was feeling a bit nauseous with the other medication so I stopped taking it. And since I didn't know what it was for I forgot about it. But know that I learned that I should take a Statin I wanted to make sure I took one that did not upset my stomach. | Yes, I check my blood sugar more frequently. I even brought it with me during my vacation so that I can check my sugars here. I've never done that before. I also have been walking a lot during my trip so that I don't get lazy and stop. |
|
82-year old Caucasian female Baseline Activation Level: 2 6-month Follow-Up Level: 4 PAM Score: +36.4 points |
No | Yes, my doctor decided to put me on cholesterol medication. I told her I would, but that I wanted the lowest dosage and I made sure I found out what all side effects were before I started taking it. I told her I would take it for three months and see how much it lowered my cholesterol; if it did not lower it significantly then I would stop taking it and ask for something else. | I exercise a little more. Also, I make sure I take my list of questions to all my appointments. |
|
87 year-old Caucasian male Baseline Activation Level: 4 6-month Follow-Up Level: 4 PAM Score: No change |
No | No | I learned that it's alright to disagree with my doctor and to ask him questions if I'm not OK with what he wants me to do. |
DISCUSSION
Despite the potential of patient activation to improve chronic disease outcomes, widespread implementation of patient activation interventions has not occurred6,9. The present study provides important pilot data in support of further investigation of interventions in community settings to activate seniors with a significant chronic disease burden.
Our intervention targeted a population with an average of 2 chronic diseases, reaching them in a setting without the time pressures inherent to primary care. The results suggest that our intervention was successful in increasing participants’ activation level and that initial increases may be self-reinforcing. While the average activation level of those who participated in 2 or fewer group screenings remained largely unchanged over time, participants who attended 3 or more group screenings increased their activation from post-intervention to 6-month follow-up. The greatest increases in activation were observed among those with the lowest level of activation at baseline. Although overall self-reported physical activity decreased from baseline, which could reflect seasonal variation in physical activity21, participants who attended 3 or more group screenings reported significantly more minutes per week walking at both follow-up points and more minutes of vigorous physical activity per week at 6-month follow-up. Our results suggest that participants who attended 3 or more group screenings had better health-related quality of life at 6-month follow-up, both in terms of physical and mental health. However, these findings should be interpreted with caution. First, although these differences were non-significant, participants who attended more group screenings reported more physical activity at baseline, which could impact the physical component scores. Second, the difference in mental health component scores appeared to result from a drop in scores among those attending fewer screenings, while those attending 3 or more screenings appeared to maintain their gains from baseline. Further research is needed to better understand the relationship between increased activation and quality of life.
The survey items assessing participants’ subjective perceptions of change as well as the open-ended responses from participants reinforce the overall conclusions suggested by these data. Our data also suggest that participants who viewed the program on advance directives were more likely to complete an advance directive or intend to do so in the near future. The important question that remains open is whether the self-reported changes observed in this study would translate into improvements on relevant objective measures. Future studies will need to examine whether an activation intervention delivered in a community setting can lead to improvements in measures such as hemoglobin A1c, lipids or blood pressure or physical activity assessed with pedometers.
Interestingly, none of the seniors in either center viewed the video programs outside of group screenings. Although a television and DVD player were provided to both senior centers to enable individual viewing, seniors showed little interest in doing this. This suggests that a facilitator led group activity is needed to engage seniors with the topic of chronic disease self-management.
There are several additional important limitations to this study. First, the seniors who participated in our study constituted a small sample of the population served by the respective centers and it is unclear whether they were representative. Our follow-up completion rate suggests that our participants were highly motivated; however, the follow-up completion rates were equally high regardless of how many group screenings participants attended. Because we delivered our intervention in community senior centers, we decided that randomization at the individual level was not feasible. Moreover, we decided that we could not withhold the intervention from one center while delivering it in the other, as we were concerned that we would be unable to recruit and retain participants for a study in which there was no intervention. The financial incentive was clearly effective in increasing participation in group screenings. However, our sample was not adequately powered to demonstrate differences for each primary outcome variable at the center level. As a result, our primary independent variable – repeated exposure to group screenings – was not randomly allocated, thereby introducing a risk of selection bias into our quasi-experimental study design. Our baseline data suggest that participants who attended 3 or more group screenings were not differentially activated to begin with; however they were younger and more likely to be female. Including these confounding variables in our models did not alter our findings, but it is unclear if some other important unmeasured variable may account for who attended 3 or more group screenings. These participants may have been more enthusiastic about our intervention to begin with or may have held beliefs that influenced their willingness to participate in multiple group screenings; which in turn could be related to their propensity to become more activated. Our decision to divide participants into two groups based on how many group screenings they attended was premised on the fact that repeated exposure to a concept is necessary to increase learning. But we cannot rule out potential self-selection bias in the composition of these two groups. Further research is needed to determine whether similar effects would be observed if participants were randomly assigned to repeated exposure to group screenings. Our sample also did not have sufficient power to detect dose-response effects; hence future studies should examine what the optimal “dose” of intervention to increase patient activation is.
The video programs used in our intervention focused on multiple chronic conditions that are common among older adults. Despite the focus on multiple conditions, each program reinforced the message that active self-care improves chronic condition outcomes. It is unclear whether the intervention effects would be greater if we had focused on only one specific condition. Alternatively, by including multiple conditions the message that active management is important may appear more generalized and therefore more effective in changing beliefs – the most basic cognitive determinants of behavior22 – about the role of the patient in influencing chronic disease outcomes. Our study design also precludes us from determining whether the observed effects can be attributed to the content of the video programs, the role of the facilitator that led the group screenings, or both. A more complex experimental design is necessary to answer this question, although we speculate that both are necessary. One the one hand the facilitator played an important role in encouraging the seniors to discuss the video programs, at the same time reinforcing the central message of the programs. On the other hand, we suspect that initially conveying the message of increasing self-management is more effective using an engaging video program versus, for example, using a lecture format.
Future studies using a more rigorous randomized design will need to confirm the intervention effects observed in this study and whether these will translate into meaningful improvements in clinical outcome measures. Nevertheless, our findings suggest a potentially promising intervention to activate seniors that warrants further investigation for improving chronic disease outcomes.
ACKNOWLEDGMENTS
The authors thank the staff in the participating community senior centers for their assistance in conducting this project. This project was supported by a grant from the Foundation for Informed Medical Decision Making.
Supported by a grant from the Foundation for Informed Medical Decision Making (FIMDM). Dr. Mangione received additional support from NIH/NIA Grant P30-AG021684. Ms. Ochoa received additional support from NIH/NIA Grant P30 AG 028748. The content in this article does not necessarily represent the official views of the FIMDM, NIA or NIH.
Footnotes
Conflict of interest: Dr. Frosch serves as a consultant for the Foundation for Informed Medical Decision Making. Dr. Mangione serves as the Medical Editor for the diabetes program developed by the Foundation for Informed Medical Decision Making. Mr. Rincon and Ms. Ochoa have no conflicts of interest to report.
Dr. Frosch serves as a consultant for the Foundation for Informed Medical Decision Making. Dr. Mangione serves as the Medical Editor for the diabetes program developed by the Foundation for Informed Medical Decision Making. Mr. Rincon and Ms. Ochoa have no conflicts of interest to report.
REFERENCES
- 1.Anderson G, Horvath J. The growing burden of chronic disease in America. Public Health Rep. 2004;119:263–270. doi: 10.1016/j.phr.2004.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Department of Health and Human Services The burden of chronic diseases and their risk factors. 2004.
- 3.Weiner DK. Office management of chronic pain in the elderly. Am J Med. 2007;120:306–315. doi: 10.1016/j.amjmed.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 4.Druss BG, Marcus SC, Olfson M, et al. Comparing the national economic burden of five chronic conditions. Health Aff (Millwood ) 2001;20:233–241. doi: 10.1377/hlthaff.20.6.233. [DOI] [PubMed] [Google Scholar]
- 5.Hibbard JH, Stockard J, Mahoney ER, et al. Development of the Patient Activation Measure (PAM): Conceptualizing and measuring activation in patients and consumers. Health Serv Res. 2004;39:1005–1026. doi: 10.1111/j.1475-6773.2004.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hibbard JH, Mahoney ER, Stock R, et al. Do increases in patient activation result in improved self-management behaviors? Health Serv Res. 2007;42:1443–1463. doi: 10.1111/j.1475-6773.2006.00669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hibbard JH, Greene J, Tusler M. Improving the outcomes of disease management by tailoring care to the patient's level of activation. Am J Manag Care. 2009;15:353–360. [PubMed] [Google Scholar]
- 8.Mosen DM, Schmittdiel J, Hibbard J, et al. Is patient activation associated with outcomes of care for adults with chronic conditions? J Ambul Care Manage. 2007;30:21–29. doi: 10.1097/00004479-200701000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Hibbard JH. Using systematic measurement to target consumer activation strategies. Med Care Res Rev. 2009;66:9S–27S. doi: 10.1177/1077558708326969. [DOI] [PubMed] [Google Scholar]
- 10.Von KM, Gruman J, Schaefer J, et al. Collaborative management of chronic illness. Ann Intern Med. 1997;127:1097–1102. doi: 10.7326/0003-4819-127-12-199712150-00008. [DOI] [PubMed] [Google Scholar]
- 11.Aday RH. The evolving role of senior centers in the 21st century. In: US Senate Special Commitee on Aging, editor. Baby Boomers at the Gate. Government Printing Office; 2003. pp. 69–79. [Google Scholar]
- 12.Phelan EA, Williams B, Leveille S, et al. Outcomes of a community-based dissemination of the health enhancement program. J Am Geriatr Soc. 2002;50:1519–1524. doi: 10.1046/j.1532-5415.2002.50407.x. [DOI] [PubMed] [Google Scholar]
- 13.Block G, Miller M, Harnack L, et al. An interactive CD-ROM for nutrition screening and counseling. Am J Public Health. 2000;90:781–785. doi: 10.2105/ajph.90.5.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mangione CM, Brown A, Sarkisian C, et al. Randomized community-based intervention to improve self-management of diabetes among older African Americans and Latinos. Soc Gen Intern Med. 2007:4–28. [Google Scholar]
- 15.Powe BD, Ntekop E, Barron M. An intervention study to increase colorectal cancer knowledge and screening among community elders. Public Health Nurs. 2004;21:435–442. doi: 10.1111/j.0737-1209.2004.21507.x. [DOI] [PubMed] [Google Scholar]
- 16.Rollnick S, Miller WR, Butler CC. Motivational Interviewing in Health Care: Helping Patients Change Behavior. Guilford Press; New York, NY: 2008. [Google Scholar]
- 17.Hibbard JH, Mahoney ER, Stockard J, et al. Development and testing of a short form of the patient activation measure. Health Serv Res. 2005;40:1918–1930. doi: 10.1111/j.1475-6773.2005.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson-Kozlow M, Rock CL, Gilpin EA, et al. Validation of the WHI brief physical activity questionnaire among women diagnosed with breast cancer. Am J Health Behav. 2007;31:193–202. doi: 10.5555/ajhb.2007.31.2.193. [DOI] [PubMed] [Google Scholar]
- 19.Hays RD, Prince-Embury S, Chen H. RAND-36 Health Status Inventory. The Psychological Corporation; San Antonio, Tx: 1998. [Google Scholar]
- 20.Fischer D, Stewart AL, Bloch DA, et al. Capturing the patient's view of change as a clinical outcome measure. JAMA. 1999;282:1157–1162. doi: 10.1001/jama.282.12.1157. [DOI] [PubMed] [Google Scholar]
- 21.Pivarnik JM, Reeves MJ, Rafferty AP. Seasonal variation in adult leisure-time physical activity. Med Sci Sports Exerc. 2003;35:1004–1008. doi: 10.1249/01.MSS.0000069747.55950.B1. [DOI] [PubMed] [Google Scholar]
- 22.Fishbein M. The role of theory in HIV prevention. AIDS Care. 2000;12:273–278. doi: 10.1080/09540120050042918. [DOI] [PubMed] [Google Scholar]