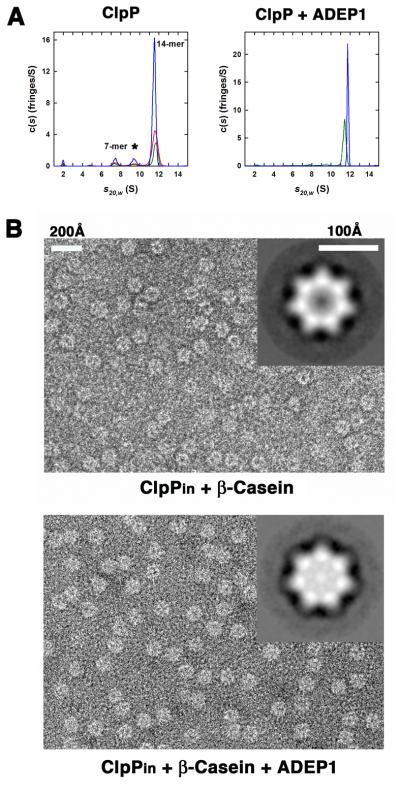
Figure 3. ADEP1 Stabilize the ClpP Tetradecamer and Promotes Substrate Translocation into its Degradation Chamber.
(A) Continuous c(s) distributions obtained from sedimentation velocity data collected at 40 krpm for ClpP in the absence of added ADEP1 (left panel). Data were collected at 101 μM (blue), 54 μM (red) and 27 μM (green) of ClpP monomer (left panel). At these concentrations, the ClpP tetradecamer (14-mer) represented the major species at approximately 81% of the total signal. Species formed from the dissociation of the tetradecamer are found at 7.5 S (~ 8% of loading signal, presumed 7-mer) and 9.4 S (~ 8% of loading signal). Traces (~ 2% of loading signal) of ClpP monomer are found at 2.0 S. Similar experiment for ClpP in the presence of added ADEP1 (right panel). Data were collected at approximately 53 μM of ClpP monomer in the presence of 0.7% (v/v) DMSO (green) or 5 equivalents of ADEP1 (blue) dissolved in DMSO. In the presence of ADEP1, data are consistent with the presence of a single ClpP tetradecamer at 11.8 S.
(B) Negative stained electron micrographs comparing ClpPin particles incubated for 2 min with β-casein in the absence (top panel) and presence of ADEP1 (bottom panel). Insets in the micrographs compare top view averages of ~500 particles of ClpPin from each sample. Less stain penetration (brighter) correlates with accumulation of β-casein inside the inner cavity. See also Figure S3.