Abstract
Background and Purpose
Ultrasound has been shown to increase rt-PA thrombolysis via stable cavitation, or sustained bubble activity, but this mechanism needs further optimization. Use of low-frequency ultrasound in combination with microbubbles stabilized against dissolution, in the form of ultrasound contrast agents, has resulted in greater lytic efficacy in vitro.
Summary of Review
This article reviews the motivation for developing ultrasound-enhanced thrombolysis, and the existing evidence for its potential as an intervention for ischemic stroke. Stable cavitation is discussed and current in vitro and ex vivo studies of bubble-mediated rt-PA clot lysis are summarized.
Conclusions
Ultrasound-driven stable cavitation nucleated by an infusion of an echo contrast agent facilitates rt-PA thrombolysis. Optimization of this gently effervescent phenomenon has the potential to reduce the morbidity and mortality of victims of ischemic stroke.
Keywords: rt-PA, ultrasound, bubbles, cavitation, thrombolysis
Introduction
The administration of recombinant tissue plasminogen activator (rt-PA) in patients with ischemic stroke is only moderately effective, and results in a 30% greater chance of little or no disability compared to placebo at 3 months.1 A 6.4% incidence of intracerebral hemorrhage (ICH) exists in patients receiving this thrombolytic therapy.1 Thus an improved intervention that could increase lytic efficacy and reduce the incidence of ICH would lead to a significant improvement in outcomes for stroke patients.
Adjuvant ultrasound has been shown to enhance rt-PA thrombolysis in vitro and in vivo.2-8 Improvement of lysis locally using an ultrasound beam may enhance the efficacy and has the potential to improve the safety of rt-PA treatment.
In vitro studies have shown that ultrasound-assisted thrombolysis is mediated by stable cavitation, or sustained bubble activity.9-11 Acoustic cavitation has been studied extensively both theoretically and experimentally by investigators in an attempt to understand the cause of ultrasound bioeffects.12-22 Acoustic cavitation is the formation and collapse of gaseous and vapor bubbles in a liquid due to an acoustic pressure field. Cavitation is generally classified into two types: stable cavitation, which results in emissions at the subharmonic and odd ultraharmonics of the main excitation frequency, and inertial cavitation, which is characterized by broadband noise emissions. When a bubble oscillates nonlinearly about its equilibrium radius, Faraday waves are excited along the bubble wall, causing subharmonic emissions.23 Broadband noise emissions are generated when bubbles undergo large radial oscillations and collapse violently.15,16,24,25 This type of bubble motion is dominated by the inertia of the surrounding fluid, hence the label “inertial” cavitation. Stable cavitation can induce microstreaming26 and inertial cavitation can cause microjetting and pitting on solid surfaces.27-29
Enhancement of rt-PA thrombolysis with low-frequency ultrasound
Low-frequency ultrasound (< 1 MHz) is a good choice for skull penetration,30 and accelerates rt-PA thrombolysis.31-33 Alexandrov et al.34 employed 2-MHz transcranial Doppler ultrasound to monitor flow in the middle cerebral artery in acute ischemic stroke patients, and observed an increase in the rate of sustained complete recanalization within two hours after the administration of rt-PA in those patients who were monitored with ultrasound. Unfortunately, another clinical trial utilizing transcranial 300-kHz ultrasound, the TRanscranial low-frequency Ultrasound-Mediated thrombolysis in Brain Ischemia (TRUMBI) trial, was ended early when 13 of 14 patients being treated with rt-PA and ultrasound had evident intracranial bleeding on MRI, compared to 5 of 12 treated with rt-PA alone, and 5 of them experienced symptomatic hemorrhage within three days of treatment.35 Later simulations showed that standing waves produced inside the skull during this trial may have produced peak rarefactional pressures in excess of 1 MPa,36 and exploration of treatments using lower amplitude insonation are warranted.
Bubble activity and thrombolysis
Injection of Definity®, an FDA-approved ultrasound contrast agent (UCA), allows blood plasma to become a good substrate for stable cavitation when exposed to low ultrasound pressure amplitudes.10 Ultraharmonic emissions can be used to monitor and measure cavitation activity, and such signals are significantly correlated with increased rt-PA lytic efficacy in vitro. The synergistic effect of sustained bubble activity has also been observed at a significantly lower concentration of rt-PA (1.4 μg/mL),10 thus providing the potential for increased safety profile of this drug clinically.
Molina et al.37 combined a galactose-based UCA and rt-PA with 2-MHz transcranial Doppler in acute ischemic stroke patients. Controls received rt-PA alone, and a third group received ultrasound and rt-PA without the UCA. The recanalization rate at two hours was significantly greater in those who received combined rt-PA, ultrasound and UCA treatment (54%) compared with rt-PA and ultrasound (41%) and rt-PA alone (24%). The long-term outcomes of each treatment were not studied. Overall, ultrasound-enhanced thrombolysis is a promising therapy for stroke, but much work remains to be done to demonstrate its clinical advantage.
Bubble-driven thrombolysis in an ex vivo artery flow system
An ex vivo artery model that incorporates physiologic flow and pressure has been developed to study cavitation-mediated drug delivery and thrombolysis.38-40 Aged porcine whole blood clots were placed into the lumina of living, excised porcine carotid arteries to simulate the human middle cerebral artery. Pooled, citrated porcine plasma flowed slowly through the artery, (0 – 8 mL/min, mean 2.7 +/− 1.8 mL/min) to simulate the reduced flow environment during ischemic stroke. Each clot was weighed before and after its 30-minute treatment to provide clot mass loss as a metric of thrombolytic efficacy. Combinations of the following treatments were applied: rt-PA (3.9 ± 2.1 μg/mL), Definity® (0.38 ± 0.21 μL/mL), and ultrasound (120-kHz continuous wave at a 0.44 MPa peak-to-peak pressure amplitude). The ultrasound was employed in an intermittent scheme so that there was periodic replenishment of Definity® microbubbles in the volume of plasma surrounding the intraluminal blood clot. Because flow in the middle cerebral artery in ischemic stroke is very low, if present,41,42 the quiescent periods were 19.5 seconds to facilitate Definity® influx between continuous wave ultrasound episodes lasting 8.5 seconds each. Cavitation activity was monitored throughout each treatment.
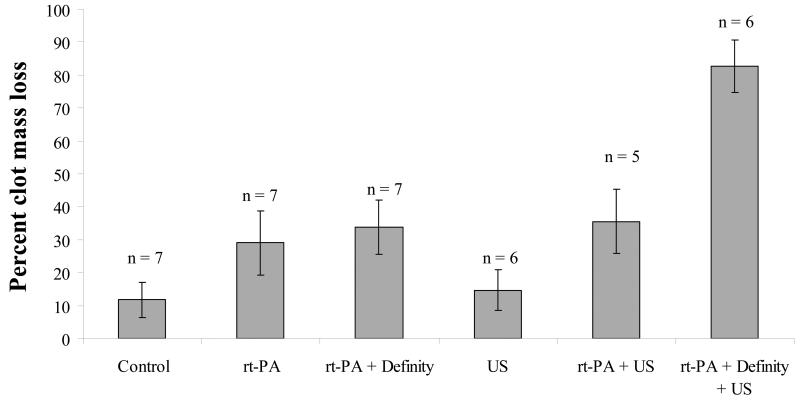
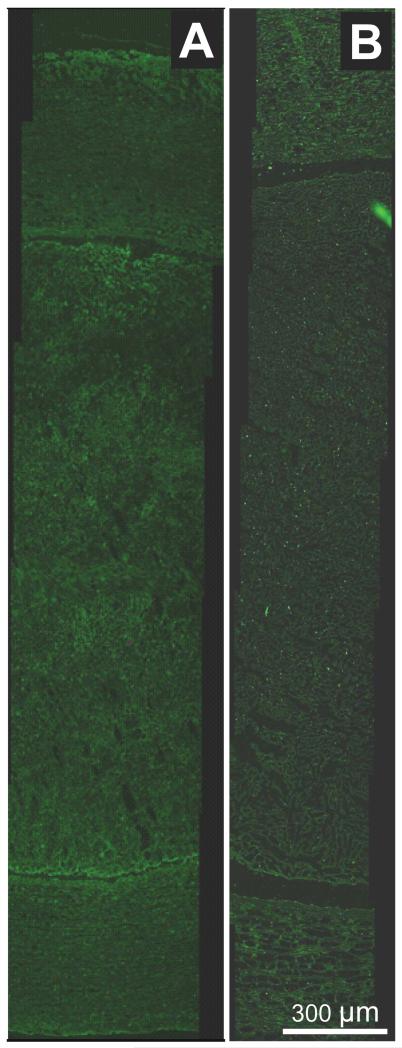
In the ex vivo flow system, porcine plasma alone (carrying 8 ng/mL of endogenous t-PA), produced a clot mass loss of 12% over a 30-minute sham treatment, as shown in Figure 1. The addition of rt-PA resulted in 29% clot mass loss. Combinations of Definity® with rt-PA, or ultrasound with rt-PA and no Definity®, did not increase mass loss over rt-PA alone. However, when Definity® and ultrasound were combined to produce stable cavitation in the presence of rt-PA, mass loss increased to 83%. The maximum mass loss achieved was limited by the physical arrangement as only 2/3 of the clot length sat within the beam of the ultrasound. No enhancement of rt-PA extravasation was noted in any of the arteries by a pathologist blinded to the exposure conditions, as shown in Figure 2.
Figure 1.
Percent of initial whole-blood clot mass removed by each 30-minute treatment within an ex vivo porcine carotid artery in flowing plasma. Error bars represent one standard deviation.
Figure 2.
Representative fluorescence images of anti-t-PA-labeled arteries with luminal clot.10,40 A – clot treated with rt-PA (3.9 ± 2.1 μg/mL) and Definity® microbubbles (0.38 ± 0.21 μL/mL) in flowing porcine plasma, with ultrasound exposure (120-kHz continuous wave at a 0.44 MPa peak-to-peak pressure amplitude). B – clot treated with rt-PA only. Note that although both arteries display abundant autofluorescence, the ultrasound-treated artery does not demonstrate enhanced rt-PA penetration into the arterial tissue.
Future Studies
A targeted thrombolytic delivery system utilizing echogenic liposomes (ELIP) is under development.43-45 Studies in vivo have demonstrated successful targeting of the ELIP to a thrombus surface using inactivated t-PA.44,46 An in vitro study demonstrated effective use of selectively tuned clinical diagnostic ultrasound to release drug from rt-PA-loaded ELIP.47,48 The rt-PA-loaded ELIP will be tested in the ex vivo artery system to determine optimal ultrasound parameters for delivery of the lytic drug and for promotion of stable cavitation to enhance efficacy. The encapsulation of a lytic combined with a cavitation nucleation agent and gas which promotes vasodilation within a lipid bilayer has the potential to restore physiologic flow with minimal exposure of the whole body to the enzyme.
Conclusions
A new treatment that limits the whole-body dose of rt-PA while maintaining its efficacy in a spatially controlled fashion could reduce the hemorrhagic complications of lytic treatment. Adjunctive use of ultrasound-driven bubble activity during intravenous rt-PA treatment has the potential to enhance thrombolytic therapy. A number of stabilized microbubble preparations, some of which are already approved by the FDA as ultrasound contrast agents, make it possible to employ low ultrasound pressure amplitudes to promote stable cavitation, thereby reducing the potential for negative ultrasonic bioeffects. Ongoing in vitro, ex vivo, and in vivo studies are aimed at developing this promising technique.
Acknowledgments and Funding
Research described here was funded by the National Institutes of Health grant numbers R01-NS047603, T32-GM063483, the Albert J. Ryan Foundation Fellowship, and the Rindsberg Memorial Fellowship.
Footnotes
The authors have no conflicts of interests or disclosures to report.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- (1).Marler JR. Tissue plasminogen activator for acute ischemic stroke. N.Engl.J.Med. 1995;333(24):1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- (2).Francis CW, Blinc A, Lee S, Cox C. Ultrasound accelerates transport of recombinant tissue plasminogen activator into clots. Ultrasound in Medicine and Biology. 1995;21(3):419–424. doi: 10.1016/0301-5629(94)00119-x. [DOI] [PubMed] [Google Scholar]
- (3).Francis CW, Onundarson PT, Carstensen EL, Blinc A, Meltzer RS, Schwarz K, Marder VJ. Enhancement of fibrinolysis in vitro by ultrasound. J.Clin.Invest. 1992;90(5):2063–2068. doi: 10.1172/JCI116088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Blinc A, Francis CW, Trudnowski JL, Carstensen EL. Characterization of ultrasound-potentiated fibrinolysis in vitro. Blood. 1993;81(10):2636–2643. [PubMed] [Google Scholar]
- (5).Behrens S, Daffertshofer M, Spiegel D, Hennerici M. Low-frequency, low-intensity ultrasound accelerates thrombolysis through the skull. Ultrasound in Medicine and Biology. 1999;25(2):269–273. doi: 10.1016/s0301-5629(98)00158-6. [DOI] [PubMed] [Google Scholar]
- (6).Akiyama M, Ishibashi T, Yamada T, Furuhata H. Low-frequency ultrasound penetrates the cranium and enhances thrombolysis in vitro. Neurosurgery. 1998;43(4):828–832. doi: 10.1097/00006123-199810000-00062. [DOI] [PubMed] [Google Scholar]
- (7).Saguchi T, Onoue H, Urashima M, Ishibashi T, Abe T, Furuhata H. Effective and Safe Conditions of Low-Frequency Transcranial Ultrasonic Thrombolysis for Acute Ischemic Stroke. Neurologic and Histologic Evaluation in a Rat Middle Cerebral Artery Stroke Model. Stroke. 2008 doi: 10.1161/STROKEAHA.107.496117. [DOI] [PubMed] [Google Scholar]
- (8).Alexandrov AV. Ultrasound identification and lysis of clots. Stroke. 2004;35(11):2722–2725. doi: 10.1161/01.STR.0000143321.37482.b3. [DOI] [PubMed] [Google Scholar]
- (9).Datta S, Coussios CC, McAdory LE, Tan J, Porter T, De Courten-Myers G, Holland CK. Correlation of cavitation with ultrasound enhancement of thrombolysis. Ultrasound in Medicine and Biology. 2006;32(8):1257–1267. doi: 10.1016/j.ultrasmedbio.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Datta S, Coussios C, Ammi AY, Mast TD, de Courten-Myers GM, Holland CK. Ultrasound-enhanced thrombolysis using Definity® as a cavitation nucleation agent. Ultrasound Med.Biol. 2008 doi: 10.1016/j.ultrasmedbio.2008.01.016. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Prokop AF, Soltani A, Roy RA. Cavitational Mechanisms in Ultrasound-Accelerated Fibrinolysis. Ultrasound in Medicine and Biology. 2007;33(6):924–933. doi: 10.1016/j.ultrasmedbio.2006.11.022. [DOI] [PubMed] [Google Scholar]
- (12).Apfel RE. Acoustic cavitation prediction. J.Acoust.Soc.Am. 1981;69(6):1624–1633. [Google Scholar]
- (13).Apfel RE. Acoustic cavitation: A possible consequence of biomedical uses of ultrasound. Br.J.Cancer. 1982;45:140–146. [PMC free article] [PubMed] [Google Scholar]
- (14).Holland CK, Apfel RE. Improved theory for the prediction of microcavitation thresholds. IEEE Trans.Ultrason.Ferroelectr.Freq.Control. 1989;36(2):204–208. doi: 10.1109/58.19152. [DOI] [PubMed] [Google Scholar]
- (15).Holland CK, Apfel RE. Thresholds for transient cavitation produced by pulsed ultrasound in a controlled nuclei environment. J.Acoust.Soc.Am. 1990;88(5):2059–2069. doi: 10.1121/1.400102. [DOI] [PubMed] [Google Scholar]
- (16).Holland CK, Deng CX, Apfel RE, Alderman JL, Fernandez LA, Taylor KJW. Direct evidence of cavitation in vivo from diagnostic ultrasound. Ultrasound in Medicine and Biology. 1996;22(7):917–925. doi: 10.1016/0301-5629(96)00083-x. [DOI] [PubMed] [Google Scholar]
- (17).Holland CK, Roy RA, Apfel RE, Crum LA. In Vitro detection of cavitation induced by a diagnostic ultrasound system. IEEE Trans.Ultrason.Ferroelectr.Freq.Control. 1992;39(1):95–101. doi: 10.1109/58.166815. [DOI] [PubMed] [Google Scholar]
- (18).Deng CX, Sieling F, Pan H, Cui J. Ultrasound-induced cell membrane porosity. Ultrasound in Medicine and Biology. 2004;30(4):519–526. doi: 10.1016/j.ultrasmedbio.2004.01.005. [DOI] [PubMed] [Google Scholar]
- (19).Atchley AA, Frizzell LA, Apfel RE, Holland CK, Madanshetty S, Roy RA. Thresholds for cavitation produced in water by pulsed ultrasound. Ultrasonics. 1988;26(5):280–285. doi: 10.1016/0041-624x(88)90018-2. [DOI] [PubMed] [Google Scholar]
- (20).Flynn HG. Generation of transient cavities in liquids by microsecond pulses of ultrasound. J.Acoust.Soc.Am. 1982;72(6):1926–1932. [Google Scholar]
- (21).Flynn HG, Church CC. Mechanism for the generation of cavitation maxima by pulsed ultrasound. J.Acoust.Soc.Am. 1984;76(2):505–512. doi: 10.1121/1.391592. [DOI] [PubMed] [Google Scholar]
- (22).Carstensen EL, Flynn HG. The potential for transient cavitation with microsecond pulses of ultrasound. Ultrasound in Medicine and Biology. 1982;8(6):L720–724. doi: 10.1016/0301-5629(82)90134-x. [DOI] [PubMed] [Google Scholar]
- (23).Phelps AD, Leighton TG. The Subharmonic Oscillations and Combination-Frequency Subharmonic Emissions from a Resonant Bubble: Their Properties and Generation Mechanisms. Acustica. 1997;83(1):59–66. [Google Scholar]
- (24).ANSI Bubble detection and Cavitation Monitoring. 2002 [Google Scholar]
- (25).Roy RA, Madanshetty SI, Apfel RE. An acoustic backscattering technique for the detection of transient cavitation produced by microsecond pulses of ultrasound. J.Acoust.Soc.Am. 1990;87(6):2451–2458. doi: 10.1121/1.399091. [DOI] [PubMed] [Google Scholar]
- (26).Miller DL. Particle gathering and microstreaming near ultrasonically activated gas-filled micropores. J.Acoust.Soc.Am. 1988;84(4):1378–1387. doi: 10.1121/1.396636. [DOI] [PubMed] [Google Scholar]
- (27).Crum LA. Cavitation microjets as a contributory mechanism for renal calculi disintegration in ESWL. J.Urol. 1988;140(6):1587–1590. doi: 10.1016/s0022-5347(17)42132-x. [DOI] [PubMed] [Google Scholar]
- (28).Bailey MR, Blackstock DT, Cleveland RO, Crum LA. Comparison of electrohydraulic lithotripters with rigid and pressure- release ellipsoidal reflectors. I. Acoustic fields. J.Acoust.Soc.Am. 1998;104(4):2517–2524. doi: 10.1121/1.423758. [DOI] [PubMed] [Google Scholar]
- (29).Bailey MR, Blackstock DT, Cleveland RO, Crum LA. Comparison of electrohydraulic lithotripters with rigid and pressure- release ellipsoidal reflectors. II. Cavitation fields. J.Acoust.Soc.Am. 1999;106(2):1149–1160. doi: 10.1121/1.427123. [DOI] [PubMed] [Google Scholar]
- (30).Ammi AY, Mast TD, Huang I, Abruzzo TA, Coussios C, Shaw GJ, Holland CK. Characterization of the ultrasound propagation through ex-vivo human temporal bone. Ultrasound Med.Biol. 2008 doi: 10.1016/j.ultrasmedbio.2008.02.012. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Behrens S, Spengos K, Daffertshofer M, Schroeck H, Dempfle CE, Hennerici M. Transcranial ultrasound-improved thrombolysis: Diagnostic vs. therapeutic ultrasound. Ultrasound in Medicine and Biology. 2001;27(12):1683–1689. doi: 10.1016/s0301-5629(01)00481-1. [DOI] [PubMed] [Google Scholar]
- (32).Behrens S, Spengos K, Daffertshofer M, Wirth S, Hennerici M. Potential use of therapeutic ultrasound in ischemic stroke treatment. Echocardiography. 2001;18(3):259–263. doi: 10.1046/j.1540-8175.2001.00259.x. [DOI] [PubMed] [Google Scholar]
- (33).Nedelmann M, Eicke BM, Lierke EG, Heimann A, Kempski O, Hopf HC. Low-frequency ultrasound induces nonenzymatic thrombolysis in vitro. Journal of Ultrasound in Medicine. 2002;21(6):649–656. doi: 10.7863/jum.2002.21.6.649. [DOI] [PubMed] [Google Scholar]
- (34).Alexandrov AV, Wojner AW, Grotta JC. CLOTBUST: Design of a Randomized Trial of Ultrasound-Enhanced Thrombolysis for Acute Ischemic Stroke. Journal of Neuroimaging. 2004;14(2):108–112. [PubMed] [Google Scholar]
- (35).Daffertshofer M, Gass A, Ringleb P, Sitzer M, Sliwka U, Els T, Sedlaczek O, Koroshetz WJ, Hennerici MG. Transcranial low-frequency ultrasound-mediated thrombolysis in brain ischemia: Increased risk of hemorrhage with combined ultrasound and tissue plasminogen activator - Results of a phase II clinical trial. Stroke. 2005;36(7):1441–1446. doi: 10.1161/01.STR.0000170707.86793.1a. [DOI] [PubMed] [Google Scholar]
- (36).Baron C, Aubry JF, Tanter M, Meairs S, Fink M. Simulation of intracranial acoustic fields in clinical trials of sonothrombolysis. Ultrasound Med.Biol. 2009;35(7):1148–1158. doi: 10.1016/j.ultrasmedbio.2008.11.014. [DOI] [PubMed] [Google Scholar]
- (37).Molina CA, Ribo M, Rubiera M, Montaner J, Santamarina E, Delgado-Mederos R, Arenillas JF, Huertas R, Purroy F, Delgado P, Alvarez-Sabi?n J. Microbubble administration accelerates clot lysis during continuous 2-MHz ultrasound monitoring in stroke patients treated with intravenous tissue plasminogen activator. Stroke. 2006;37(2):425–429. doi: 10.1161/01.STR.0000199064.94588.39. [DOI] [PubMed] [Google Scholar]
- (38).Hitchcock KE, Caudell DN, Sutton JT, Klegerman ME, Vela D, Pyne-Geithman GJ, Abruzzo T, Cyr PEP, Geng Y, McPherson DD, Holland CK. Ultrasound-enhanced delivery of targeted echogenic liposomes in a novel ex vivo mouse aorta model. J.Controlled Release. 2010 doi: 10.1016/j.jconrel.2010.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Hitchcock KE, Sutton JT, Caudell DN, Pyne-Geithman GJ, Klegerman ME, Huang S, Vela D, McPherson DD, Holland CK. Delivery of targeted echogenic liposomes in an ex vivo mouse aorta model. J Acoust Soc Am. 2009;125 doi: 10.1016/j.jconrel.2010.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Hitchcock KE. Ultrasound-enhanced drug delivery in a perfused ex vivo artery model. 2010 [Google Scholar]
- (41).Morawetz RB, DeGirolami U, Ojemann RG. Cerebral blood flow determined by hydrogen clearance during middle cerebral artery occlusion in unanesthetized monkeys. Stroke. 1978;9(2):143–149. doi: 10.1161/01.str.9.2.143. [DOI] [PubMed] [Google Scholar]
- (42).Shishido F, Uemura K, Inugami A. Cerebral circulation and metabolism in cerebral infarction of middle cerebral artery territory. A positron CT study with HEADTOME III and 15O labeled gases. KAKUIGAKU. 1986;23(2):123–134. [PubMed] [Google Scholar]
- (43).Hamilton A, Rabbat M, Jain P, Belkind N, Huang SL, Nagaraj A, Klegerman M, Macdonald R, McPherson DD. A physiologic flow chamber model to define intravascular ultrasound enhancement of fibrin using echogenic liposomes. Invest.Radiol. 2002;37(4):215–221. doi: 10.1097/00004424-200204000-00007. [DOI] [PubMed] [Google Scholar]
- (44).Klegerman ME, Zou Y, McPherson DD. Fibrin-targeting of echogenic liposomes with inactivated tissue plasminogen activator. J.Liposome Res. 2008 doi: 10.1080/08982100802118482. In press. [DOI] [PubMed] [Google Scholar]
- (45).Klegerman ME, Hamilton AJ, Huang SL, Tiukinhoy SD, Khan AA, MacDonald RC, McPherson DD. Quantitative immunoblot assay for assessment of liposomal antibody conjugation efficiency. Anal.Biochem. 2002;300(1):46–52. doi: 10.1006/abio.2001.5443. [DOI] [PubMed] [Google Scholar]
- (46).Tiukinhoy-Laing SD, Huang S, Klegerman M, Holland CK, McPherson DD. Ultrasound-facilitated thrombolysis using tissue-plasminogen activator-loaded echogenic liposomes. Thromb.Res. 119(6):777–784. doi: 10.1016/j.thromres.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Smith DAB. In vitro characterization of echogenic liposomes for ultrasonic delivery of recombinant tissue-type Plasminogen Activator (rt-PA) 2008:133. [Google Scholar]
- (48).Smith DAB, Vaidya S, Kopechek JA, Hitchcock KE, Huang SL, McPherson DD, Holland CK. Echogenic liposomes loaded with recombinant tissue-type plasminogen activatory (rt-PA) for image-guided, ultrasound-triggered drug release. Journal of the Acoustical Society of America. 2007b;122:3007. [Google Scholar]