Figure 1. Summary of available structure information on EI.
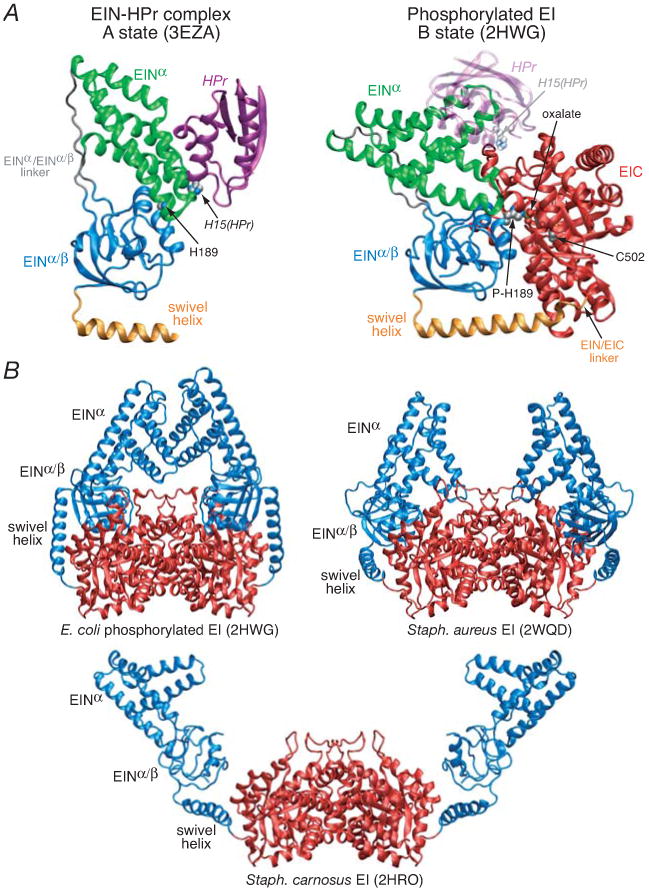
(A) Comparison of the solution NMR structure of the isolated E. coli EIN domain complexed to HPr (3EZA)13 which we refer to as the A state of the EIN domain (left panel) with that observed in the crystal structure of the phosphorylated EI intermediate from E. coli (2HWG)18 which we refer to as the B state of the EIN domain (right panel), displayed in the same orientation of the EINα/β subdomain. Only a single subunit of the phosphorylated EI dimer is shown. The structures are depicted as ribbon diagrams with the EINα and EIN α/β subdomains shown in green and blue, respectively, the swivel helix connecting the EIN and EIC domains in orange, the EIC domain in red, and HPr in purple (left panel) and transparent purple (right panel). His189 (located in the EINα/β subdomain; left panel), phospho-His189 (right panel), Cys502 (right panel), the oxalate anion (right panel) and His15 of HPr are shown as space-filling models color-coded according to atom type. HPr bound to the EINα subdomain in the same orientation as in the EIN-HPr complex is shown as a transparent ribbon in the right panel to demonstrate that the HPr binding site is available in the phosporylated EI intermediate (B state) and that there are no steric clashes between HPr and the EIC domain in this conformation of EI. (B) Comparison of the crystal structures of the phosphorylated EI intermediate from E. coli (2HWG)18 and free EI from Staph. aureus (2WQD)17 and Staph. carnosus (2HRO),16 with the EIN and EIC domains shown in blue and red, respectively. Note that the orientation of the EINα and EINα/β subdomains in isolated EIN (both in solution12,13 and in the crystal state11) is the same as that seen in the crystal structure of free EI from Staph. aureus (2WQD17). The EINα/β subdomain in Staph. carnosus EI16 is partially disordered with some regions not visible in the electron density map. The structure of the EIC dimerization domain in all three EI crystal structures is essentially the same (Cα rms differences of less than 1 Å).
