Figure 6.
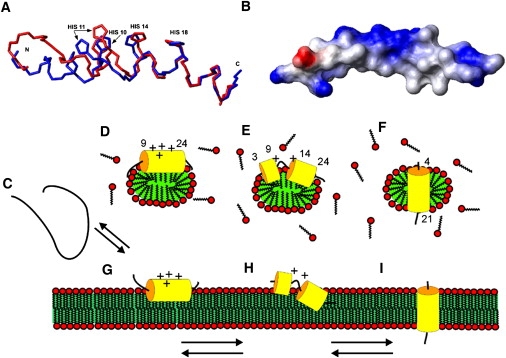
(A) Backbone traces of the mean structures of LAH4 showing the histidine heavy atoms mostly affected by pH changes. The mean structure of the peptide at pH 6.1 is displayed in blue, and the red trace represents the mean structure at pH 4.1. The superposition of both structures is based on the helical region encompassing the region between H14 and L23. (B) Space-filling model of the structure obtained at pH 6.1 and 317 K, representing the positive surface charges of the polypeptide in blue and the negative charges in red. The C-terminus is shown to the left. (C) Model representations of the LAH4 peptide in solution (random coil and monomeric at low pH (22,23)), of the structures in micellar environments (D–F) and in lipid bilayers (G–I). The surface-oriented topology at acidic pH (D and G), the transition state (E and H), and the transmembrane alignments at pH > 7 are shown (F and I) as measured in oriented phospholipid bilayers (G–I) (16) or by analogy (D–F). Panels D–F summarize the structural features observed in this work, whereas panels G–I combine these findings with the membrane topology obtained from oriented phospholipid bilayers (16,18).
