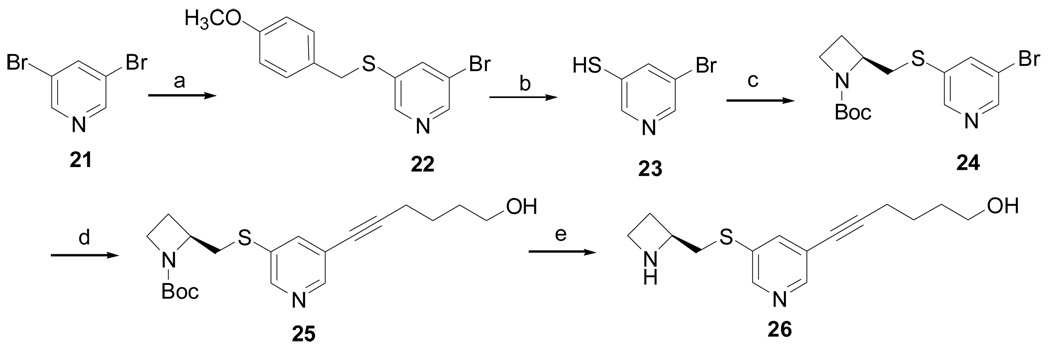
Scheme 3a.
a Reagents and conditions: (a) NaH (60%), p-MeOC6H4CH2SH, DMF, 10 h, 69%; (b) m-cresol, TFA, reflux, 24 h, 53%; (c) 2-(toluene-4-sulfonyloxymethyl)azetidine-1-carboxylic acid tert-butyl ester, K2CO3, DMF, 12 h, 50%; (d) Pd(PPh3)2Cl2, PPh3, CuI, Et3N, 5-hexyn-1-ol, 12 h, 84%; (e) 4M HCl/dioxane, 4 h, 50%.