Table 6.
Inhibitory potency of 51 GSK-3β inhibitors included in this study.
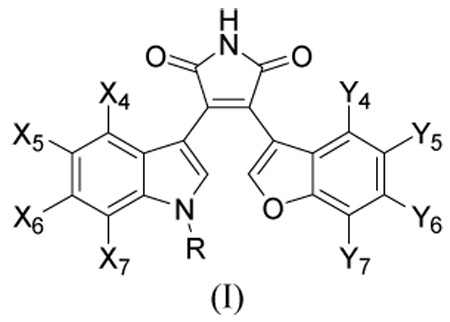 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| pIC50 | |||||||||
| IC50 | CoMFA (BM1) |
CoMSIA (BM1) |
|||||||
| X | Y | R | (nM) | Obs | Cala | Deva | Calb | Devb | |
| 1 | 7-CH2OMe | H | CH3 | 0.12 | 9.921 | 9.076 | 0.845 | 8.663 | 1.258 |
| 2 | 5-F | 6-CH2OH | CH3 | 0.35 | 9.456 | 9.114 | 0.342 | 8.960 | 0.496 |
| 3 | 5-Br | 6-CH2OH | CH3 | 0.51 | 9.292 | 9.196 | 0.096 | 8.958 | 0.334 |
| 4 | 5-F, 6-Cl | 6-CH2OH | CH3 | 0.95 | 9.022 | 8.409 | 0.613 | 8.588 | 0.434 |
| 5 | 5-Br | H | (CH2)3OH | 1.60 | 8.796 | 8.305 | 0.491 | 8.221 | 0.575 |
| 6 | 5-F | 6-OH | CH3 | 3.50 | 8.456 | 8.598 | −0.142 | 7.740 | 0.716 |
| 7 | 7-CH2OH | 6-CH2OH | CH3 | 5.10 | 8.292 | 9.305 | −1.013 | 9.475 | −1.183 |
| 8 | 7-CH2OH | H | CH3 | 5.40 | 8.268 | 8.371 | −0.103 | 8.442 | −0.174 |
| 9 | 5-Br | H | CH3 | 7.00 | 8.155 | 7.578 | 0.577 | 7.602 | 0.553 |
| 10 | 5-Br | 7-OCH3 | CH3 | 7.50 | 8.125 | 7.182 | 0.943 | 7.086 | 1.039 |
| 11 | 5-CN | 6-CH2OH | CH3 | 13.20 | 7.879 | 8.495 | −0.616 | 8.188 | −0.309 |
| 12 | 6-OH | 5-F | CH3 | 14.00 | 7.854 | 6.916 | 0.938 | 6.739 | 1.115 |
| 13 | 6-OH | H | CH3 | 15.00 | 7.824 | 7.601 | 0.223 | 7.581 | 0.243 |
| 14 | 5-F | CH3 | 22.60 | 7.646 | 6.970 | 0.676 | 6.733 | 0.913 | |
| 15 | 5-F | 6-CH2OCH3 | CH3 | 23.80 | 7.623 | 7.848 | −0.225 | 8.190 | −0.567 |
| 16 | 5-Br | CH3 | 25.30 | 7.597 | 7.913 | −0.316 | 7.647 | −0.050 | |
| 17 | 5-F | H | CH3 | 26.00 | 7.585 | 7.209 | 0.376 | 7.527 | 0.058 |
| 18 | 5-I | H | CH3 | 34.50 | 7.462 | 7.410 | 0.052 | 7.658 | −0.196 |
| 19 | H | H | CH3 | 35.00 | 7.456 | 6.911 | 0.545 | 7.038 | 0.418 |
| 20 | 5-Cl | 5-F | CH3 | 42.00 | 7.377 | 7.099 | 0.278 | 7.069 | 0.308 |
| 21 | 5-Br | CH3 | 48.30 | 7.316 | 7.350 | −0.034 | 7.469 | −0.153 | |
| 22 | 7-OH | H | CH3 | 55.00 | 7.260 | 7.326 | −0.066 | 7.764 | −0.504 |
| 23 | 5-,7-di-Br | 7-OCH3 | CH3 | 88.70 | 7.052 | 7.226 | −0.174 | 7.205 | −0.153 |
| 24 | 5-F, 6-Cl | 7-OCH3 | CH3 | 114.00 | 6.943 | 6.424 | 0.519 | 6.706 | 0.237 |
| 25 | 5-OCH3 | H | CH3 | 125.00 | 6.903 | 7.148 | −0.245 | 6.862 | 0.041 |
| 26 | 5-CN | 5,6-di-F | 131.00 | 6.883 | 7.196 | −0.313 | 6.825 | 0.058 | |
| 27 | 6-OBn | 5-F | CH3 | 160.00 | 6.796 | 6.880 | −0.084 | 6.026 | 0.770 |
| 28 | H | 7-OCH3 | CH3 | 180.00 | 6.745 | 6.381 | 0.364 | 6.696 | 0.049 |
| 29 | 5-I | 5-F | CH3 | 180.00 | 6.745 | 7.505 | −0.760 | 7.290 | −0.545 |
| 30 | 5-F, 6-Cl | H | CH3 | 184.00 | 6.735 | 6.661 | 0.074 | 7.244 | −0.509 |
| 31 | 5-OBn | H | (CH2)3OH | 220.00 | 6.658 | 7.175 | −0.517 | 7.113 | −0.455 |
| 32 | 7-OBn | H | CH3 | 220.00 | 6.658 | 6.918 | −0.260 | 7.071 | −0.413 |
| 33 | 5-OCH3, 6-I | H | CH3 | 223.00 | 6.652 | 6.703 | −0.051 | 6.948 | −0.296 |
| 34 | 5-cyclopropyl | H | CH3 | 235.00 | 6.629 | 6.495 | 0.134 | 6.719 | −0.090 |
| 35 | 5-F, 6-I | 7-OCH3 | CH3 | 247.00 | 6.607 | 6.533 | 0.074 | 6.736 | −0.129 |
| 36 | benzo[g] | 5-,6-di-F | CH3 | 314.00 | 6.503 | 6.134 | 0.369 | 6.354 | 0.149 |
| 37 | 5-Br | 6-O-(p-CH3O)-Bn | CH3 | 335.00 | 6.475 | 6.160 | 0.315 | 5.952 | 0.523 |
| 38 | 5-F | H | H | 360.00 | 6.444 | 6.570 | −0.126 | 6.873 | −0.429 |
| 39 | 5-OCH3, 6-Cl | H | CH3 | 440.00 | 6.357 | 6.536 | −0.179 | 6.513 | −0.156 |
| 40 | H | 5-Br | CH3 | 550.00 | 6.260 | 6.811 | −0.551 | 7.020 | −0.760 |
| 41 | H | 5-F | H | 670.00 | 6.174 | 7.120 | −0.946 | 7.187 | −1.013 |
| 42 | 5-OH | H | CH3 | 690.00 | 6.161 | 7.112 | −0.951 | 7.085 | −0.924 |
| 43 | 5,6-Methylenedioxy | 5-F | CH3 | 708.00 | 6.150 | 6.359 | −0.209 | 6.228 | −0.078 |
| 44 | 6-CF3 | 7-OCH3 | CH3 | 831.00 | 6.080 | 5.933 | 0.147 | 6.486 | −0.406 |
| 45 | 5-F, 6-Cl | 6-OCH3 | CH3 | 866.00 | 6.062 | 6.304 | −0.242 | 6.384 | −0.322 |
| 46 | 6-OBn | H | CH3 | 900.00 | 6.046 | 6.195 | −0.149 | 5.968 | 0.078 |
| 47 | 5-F, 6-Cl- | CH3 | 1040.00 | 5.983 | 6.011 | −0.028 | 6.034 | −0.051 | |
| 48 | 5-Morpholine-4-yl | H | CH3 | 1304.00 | 5.885 | 5.670 | 0.215 | 5.771 | 0.114 |
| 49 | 5-OBn | H | H | 1650.00 | 5.783 | 6.074 | −0.291 | 5.878 | −0.095 |
| 50 | 5-F, 6-Cl- | CH3 | 4092.00 | 5.388 | 5.800 | −0.412 | 5.366 | 0.022 | |
| 51 | 5-F, 6-p-Cl-Ph | 7-OCH3 | CH3 | 7000.00 | 5.155 | 5.359 | −0.204 | 5.694 | −0.539 |
Calculated using the three-component CoMFA model (eq 1) derived from 51 compounds using the binding mode 1.
Calculated using the three-component CoMSIA model (eq 3) derived from 51 compounds using binding mode 1.
