Abstract
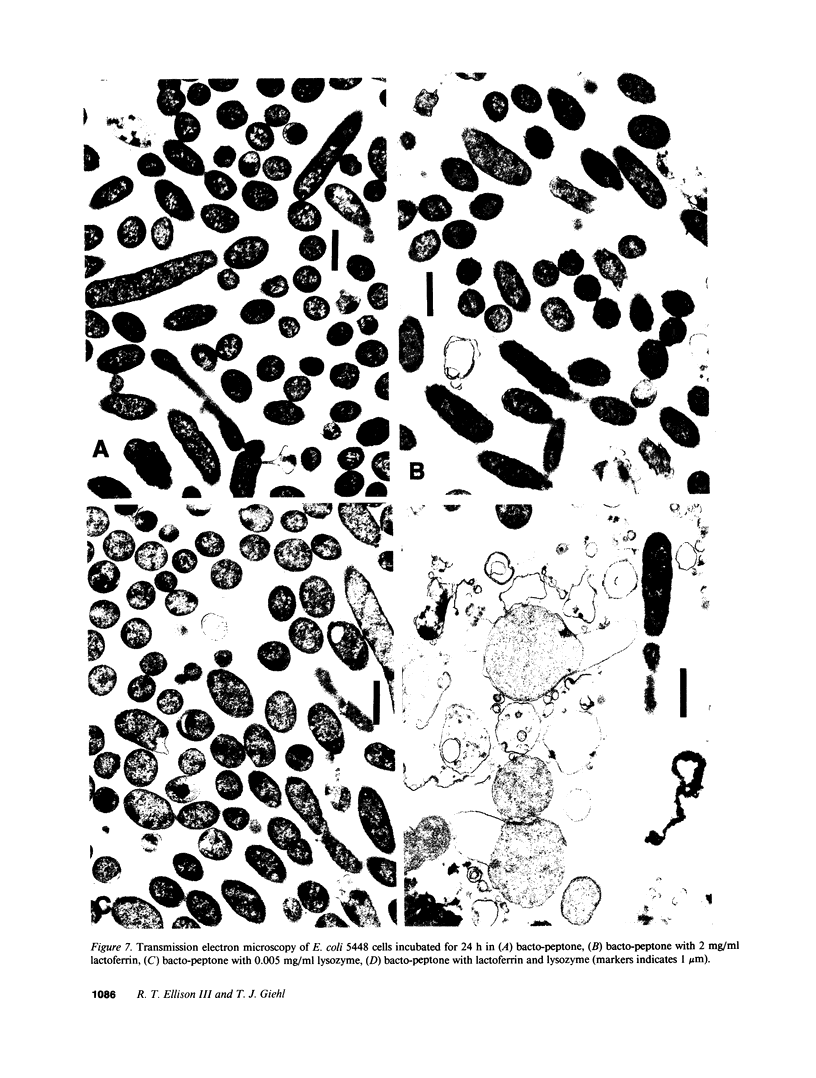
Although lactoferrin has antimicrobial activity, its mechanism of action is not full defined. Recently we have shown that the protein alters the Gram-negative outer membrane. As this membrane protects Gram-negative cells from lysozyme, we have studied whether lactoferrin's membrane effect could enhance the antibacterial activity of lysozyme. We have found that while each protein alone is bacteriostatic, together they can be bactericidal for strains of V. cholerae, S. typhimurium, and E. coli. The bactericidal effect is dose dependent, blocked by iron saturation of lactoferrin, and inhibited by high calcium levels, although lactoferrin does not chelate calcium. Using differing media, the effect of lactoferrin and lysozyme can be partially or completely inhibited; the degree of inhibition correlating with media osmolarity. Transmission electron microscopy shows that E. coli cells exposed to lactoferrin and lysozyme at 40 mOsm become enlarged and hypodense, suggesting killing through osmotic damage. Dialysis chamber studies indicate that bacterial killing requires direct contact with lactoferrin, and work with purified LPS suggests that this relates to direct LPS-binding by the protein. As lactoferrin and lysozyme are present together in high levels in mucosal secretions and neutrophil granules, it is probable that their interaction contributes to host defense.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson B. F., Baker H. M., Norris G. E., Rice D. W., Baker E. N. Structure of human lactoferrin: crystallographic structure analysis and refinement at 2.8 A resolution. J Mol Biol. 1989 Oct 20;209(4):711–734. doi: 10.1016/0022-2836(89)90602-5. [DOI] [PubMed] [Google Scholar]
- Anderson B. F., Baker H. M., Norris G. E., Rumball S. V., Baker E. N. Apolactoferrin structure demonstrates ligand-induced conformational change in transferrins. Nature. 1990 Apr 19;344(6268):784–787. doi: 10.1038/344784a0. [DOI] [PubMed] [Google Scholar]
- Arnold R. R., Brewer M., Gauthier J. J. Bactericidal activity of human lactoferrin: sensitivity of a variety of microorganisms. Infect Immun. 1980 Jun;28(3):893–898. doi: 10.1128/iai.28.3.893-898.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold R. R., Cole M. F., McGhee J. R. A bactericidal effect for human lactoferrin. Science. 1977 Jul 15;197(4300):263–265. doi: 10.1126/science.327545. [DOI] [PubMed] [Google Scholar]
- Bennett R. M., Bagby G. C., Davis J. Calcium-dependent polymerization of lactoferrin. Biochem Biophys Res Commun. 1981 Jul 16;101(1):88–95. doi: 10.1016/s0006-291x(81)80014-9. [DOI] [PubMed] [Google Scholar]
- Bezwoda W. R., Mansoor N. Lactoferrin from human breast milk and from neutrophil granulocytes. Comparative studies of isolation, quantitation, characterization and iron binding properties. Biomed Chromatogr. 1989 May;3(3):121–126. doi: 10.1002/bmc.1130030307. [DOI] [PubMed] [Google Scholar]
- Blaser M. J., Hopkins J. A., Berka R. M., Vasil M. L., Wang W. L. Identification and characterization of Campylobacter jejuni outer membrane proteins. Infect Immun. 1983 Oct;42(1):276–284. doi: 10.1128/iai.42.1.276-284.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortner C. A., Miller R. D., Arnold R. R. Bactericidal effect of lactoferrin on Legionella pneumophila. Infect Immun. 1986 Feb;51(2):373–377. doi: 10.1128/iai.51.2.373-377.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brade L., Brandenburg K., Kuhn H. M., Kusumoto S., Macher I., Rietschel E. T., Brade H. The immunogenicity and antigenicity of lipid A are influenced by its physicochemical state and environment. Infect Immun. 1987 Nov;55(11):2636–2644. doi: 10.1128/iai.55.11.2636-2644.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullen J. J., Rogers H. J., Griffiths E. Role of iron in bacterial infection. Curr Top Microbiol Immunol. 1978;80:1–35. doi: 10.1007/978-3-642-66956-9_1. [DOI] [PubMed] [Google Scholar]
- Bullen J. J. The significance of iron in infection. Rev Infect Dis. 1981 Nov-Dec;3(6):1127–1138. doi: 10.1093/clinids/3.6.1127. [DOI] [PubMed] [Google Scholar]
- Cohen M. S., Britigan B. E., French M., Bean K. Preliminary observations on lactoferrin secretion in human vaginal mucus: variation during the menstrual cycle, evidence of hormonal regulation, and implications for infection with Neisseria gonorrhoeae. Am J Obstet Gynecol. 1987 Nov;157(5):1122–1125. doi: 10.1016/s0002-9378(87)80274-0. [DOI] [PubMed] [Google Scholar]
- Curtis N. A., Eisenstadt R. L., East S. J., Cornford R. J., Walker L. A., White A. J. Iron-regulated outer membrane proteins of Escherichia coli K-12 and mechanism of action of catechol-substituted cephalosporins. Antimicrob Agents Chemother. 1988 Dec;32(12):1879–1886. doi: 10.1128/aac.32.12.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalmastri C., Valenti P., Visca P., Vittorioso P., Orsi N. Enhanced antimicrobial activity of lactoferrin by binding to the bacterial surface. Microbiologica. 1988 Jul;11(3):225–230. [PubMed] [Google Scholar]
- Ellison R. T., 3rd, Giehl T. J., LaForce F. M. Damage of the outer membrane of enteric gram-negative bacteria by lactoferrin and transferrin. Infect Immun. 1988 Nov;56(11):2774–2781. doi: 10.1128/iai.56.11.2774-2781.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison R. T., 3rd, LaForce F. M., Giehl T. J., Boose D. S., Dunn B. E. Lactoferrin and transferrin damage of the gram-negative outer membrane is modulated by Ca2+ and Mg2+. J Gen Microbiol. 1990 Jul;136(7):1437–1446. doi: 10.1099/00221287-136-7-1437. [DOI] [PubMed] [Google Scholar]
- Ellison R. T., 3rd, Luo Q., Reller L. B. Enhancement of the activity of cefotaxime by iron-binding proteins. J Antimicrob Chemother. 1990 Mar;25(3):479–481. doi: 10.1093/jac/25.3.479. [DOI] [PubMed] [Google Scholar]
- Finkelstein R. A., Sciortino C. V., McIntosh M. A. Role of iron in microbe-host interactions. Rev Infect Dis. 1983 Sep-Oct;5 (Suppl 4):S759–S777. doi: 10.1093/clinids/5.supplement_4.s759. [DOI] [PubMed] [Google Scholar]
- Goldman R. C., Leive L. Heterogeneity of antigenic-side-chain length in lipopolysaccharide from Escherichia coli 0111 and Salmonella typhimurium LT2. Eur J Biochem. 1980;107(1):145–153. doi: 10.1111/j.1432-1033.1980.tb04635.x. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Wong P. G. Compounds which increase the permeability of the Pseudomonas aeruginosa outer membrane. Antimicrob Agents Chemother. 1984 Jul;26(1):48–52. doi: 10.1128/aac.26.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock P. J. Aberrant migration of lipopolysaccharide in sodium dodecyl sulfate/polyacrylamide gel electrophoresis. Eur J Biochem. 1983 Jul 1;133(3):685–688. doi: 10.1111/j.1432-1033.1983.tb07517.x. [DOI] [PubMed] [Google Scholar]
- Hukari R., Helander I. M., Vaara M. Chain length heterogeneity of lipopolysaccharide released from Salmonella typhimurium by ethylenediaminetetraacetic acid or polycations. Eur J Biochem. 1986 Feb 3;154(3):673–676. doi: 10.1111/j.1432-1033.1986.tb09450.x. [DOI] [PubMed] [Google Scholar]
- Iacono V. J., MacKay B. J., DiRienzo S., Pollock J. J. Selective antibacterial properties of lysozyme for oral microorganisms. Infect Immun. 1980 Aug;29(2):623–632. doi: 10.1128/iai.29.2.623-632.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner K. A., Goldman R., Schmetz M., Berger M., Hammer C. H., Frank M. M., Leive L. A quantitative analysis of C3 binding to O-antigen capsule, lipopolysaccharide, and outer membrane protein of E. coli 0111B4. J Immunol. 1984 Jan;132(1):369–375. [PubMed] [Google Scholar]
- Jollès P., Jollès J. What's new in lysozyme research? Always a model system, today as yesterday. Mol Cell Biochem. 1984 Sep;63(2):165–189. doi: 10.1007/BF00285225. [DOI] [PubMed] [Google Scholar]
- Labischinski H., Barnickel G., Bradaczek H., Naumann D., Rietschel E. T., Giesbrecht P. High state of order of isolated bacterial lipopolysaccharide and its possible contribution to the permeation barrier property of the outer membrane. J Bacteriol. 1985 Apr;162(1):9–20. doi: 10.1128/jb.162.1.9-20.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B. C., Bryan L. E. Identification and comparative analysis of the lactoferrin and transferrin receptors among clinical isolates of gonococci. J Med Microbiol. 1989 Mar;28(3):199–204. doi: 10.1099/00222615-28-3-199. [DOI] [PubMed] [Google Scholar]
- Lehrer R. I., Barton A., Daher K. A., Harwig S. S., Ganz T., Selsted M. E. Interaction of human defensins with Escherichia coli. Mechanism of bactericidal activity. J Clin Invest. 1989 Aug;84(2):553–561. doi: 10.1172/JCI114198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Ganz T. Antimicrobial polypeptides of human neutrophils. Blood. 1990 Dec 1;76(11):2169–2181. [PubMed] [Google Scholar]
- Leive L. The barrier function of the gram-negative envelope. Ann N Y Acad Sci. 1974 May 10;235(0):109–129. doi: 10.1111/j.1749-6632.1974.tb43261.x. [DOI] [PubMed] [Google Scholar]
- MCGILVERY R. W., MOKRASCH L. C. Purification and properties of fructose-1, 6-diphosphatase. J Biol Chem. 1956 Aug;221(2):909–917. [PubMed] [Google Scholar]
- Mannion B. A., Weiss J., Elsbach P. Separation of sublethal and lethal effects of the bactericidal/permeability increasing protein on Escherichia coli. J Clin Invest. 1990 Mar;85(3):853–860. doi: 10.1172/JCI114512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez R. J., Carroll S. F. Sequential metabolic expressions of the lethal process in human serum-treated Escherichia coli: role of lysozyme. Infect Immun. 1980 Jun;28(3):735–745. doi: 10.1128/iai.28.3.735-745.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson P. L., Heremans J. F., Prignot J. J., Wauters G. Immunohistochemical localization and bacteriostatic properties of an iron-binding protein from bronchial mucus. Thorax. 1966 Nov;21(6):538–544. doi: 10.1136/thx.21.6.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur N. B., Dwarkadas A. M., Sharma V. K., Saha K., Jain N. Anti-infective factors in preterm human colostrum. Acta Paediatr Scand. 1990 Nov;79(11):1039–1044. doi: 10.1111/j.1651-2227.1990.tb11380.x. [DOI] [PubMed] [Google Scholar]
- NEWTON B. A. Site of action of polymyxin on Pseudomonas aeruginosa: antagonism by cations. J Gen Microbiol. 1954 Jun;10(3):491–499. doi: 10.1099/00221287-10-3-491. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985 Mar;49(1):1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Osserman E. F., Lawlor D. P. Serum and urinary lysozyme (muramidase) in monocytic and monomyelocytic leukemia. J Exp Med. 1966 Nov 1;124(5):921–952. doi: 10.1084/jem.124.5.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez Perez G. I., Blaser M. J. Lipopolysaccharide characteristics of pathogenic campylobacters. Infect Immun. 1985 Feb;47(2):353–359. doi: 10.1128/iai.47.2.353-359.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack C., Straley S. C., Klempner M. S. Probing the phagolysosomal environment of human macrophages with a Ca2+-responsive operon fusion in Yersinia pestis. 1986 Aug 28-Sep 3Nature. 322(6082):834–836. doi: 10.1038/322834a0. [DOI] [PubMed] [Google Scholar]
- Sanderson K. E., Ross H., Ziegler L., Mäkelä P. H. F + , Hfr, and F' strains of Salmonella typhimurium and Salmonella abony. Bacteriol Rev. 1972 Dec;36(4):608–637. doi: 10.1128/br.36.4.608-637.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schryvers A. B. Identification of the transferrin- and lactoferrin-binding proteins in Haemophilus influenzae. J Med Microbiol. 1989 Jun;29(2):121–130. doi: 10.1099/00222615-29-2-121. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Spik G., Cheron A., Montreuil J., Dolby J. M. Bacteriostasis of a milk-sensitive strain of Escherichia coli by immunoglobulins and iron-binding proteins in association. Immunology. 1978 Oct;35(4):663–671. [PMC free article] [PubMed] [Google Scholar]
- Stephens S., Dolby J. M., Montreuil J., Spik G. Differences in inhibition of the growth of commensal and enteropathogenic strains of Escherichia coli by lactotransferrin and secretory immunoglobulin A isolated from human milk. Immunology. 1980 Nov;41(3):597–603. [PMC free article] [PubMed] [Google Scholar]
- Thompson A. B., Bohling T., Payvandi F., Rennard S. I. Lower respiratory tract lactoferrin and lysozyme arise primarily in the airways and are elevated in association with chronic bronchitis. J Lab Clin Med. 1990 Feb;115(2):148–158. [PubMed] [Google Scholar]
- Vaara M., Vaara T. Polycations as outer membrane-disorganizing agents. Antimicrob Agents Chemother. 1983 Jul;24(1):114–122. doi: 10.1128/aac.24.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaara M., Vaara T. Polycations sensitize enteric bacteria to antibiotics. Antimicrob Agents Chemother. 1983 Jul;24(1):107–113. doi: 10.1128/aac.24.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenti P., Visca P., Antonini G., Orsi N., Antonini E. The effect of saturation with Zn2+ and other metal ions on the antibacterial activity of ovotransferrin. Med Microbiol Immunol. 1987;176(3):123–130. doi: 10.1007/BF00193893. [DOI] [PubMed] [Google Scholar]
- Watanabe N. A., Nagasu T., Katsu K., Kitoh K. E-0702, a new cephalosporin, is incorporated into Escherichia coli cells via the tonB-dependent iron transport system. Antimicrob Agents Chemother. 1987 Apr;31(4):497–504. doi: 10.1128/aac.31.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J., Franson C., Schmeidler K., Elsbach P. Reversible envelope effects during and after killing of Escherichia coli w by a highly-purified rabbit polymorpho-nuclear leukocyte fraction. Biochim Biophys Acta. 1976 Jun 4;436(1):154–169. doi: 10.1016/0005-2736(76)90227-3. [DOI] [PubMed] [Google Scholar]
- Weiss J., Muello K., Victor M., Elsbach P. The role of lipopolysaccharides in the action of the bactericidal/permeability-increasing neutrophil protein on the bacterial envelope. J Immunol. 1984 Jun;132(6):3109–3115. [PubMed] [Google Scholar]
- Weiss J., Victor M., Elsbach P. Role of charge and hydrophobic interactions in the action of the bactericidal/permeability-increasing protein of neutrophils on gram-negative bacteria. J Clin Invest. 1983 Mar;71(3):540–549. doi: 10.1172/JCI110798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West S. E., Sparling P. F. Response of Neisseria gonorrhoeae to iron limitation: alterations in expression of membrane proteins without apparent siderophore production. Infect Immun. 1985 Feb;47(2):388–394. doi: 10.1128/iai.47.2.388-394.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson R. G., Gemski P., Jr, Stocker B. A. Non-smooth mutants of Salmonella typhimurium: differentiation by phage sensitivity and genetic mapping. J Gen Microbiol. 1972 May;70(3):527–554. doi: 10.1099/00221287-70-3-527. [DOI] [PubMed] [Google Scholar]
- Wollenweber H. W., Morrison D. C. Synthesis and biochemical characterization of a photoactivatable, iodinatable, cleavable bacterial lipopolysaccharide derivative. J Biol Chem. 1985 Dec 5;260(28):15068–15074. [PubMed] [Google Scholar]
- Wright D. G., Gallin J. I. Secretory responses of human neutrophils: exocytosis of specific (secondary) granules by human neutrophils during adherence in vitro and during exudation in vivo. J Immunol. 1979 Jul;123(1):285–294. [PubMed] [Google Scholar]
- Zimelis V. M., Jackson G. G. Activity of aminoglycoside antibiotics aganst Pseudomonas aeruginosa: specificity and site of calcium and magnesium antagonism. J Infect Dis. 1973 Jun;127(6):663–669. doi: 10.1093/infdis/127.6.663. [DOI] [PubMed] [Google Scholar]