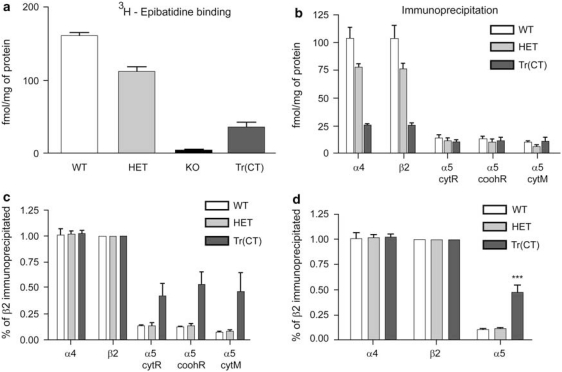
Figure 5.
Quantitative immunoprecipitation of thalamic-epibatidine binding sites with nAChR subunit-specific antibodies indicates a preferential recovery of α5 containing nAChRs in tr(CT) mice. (a) [3H]-epibatidine thalamic binding in wild type (WT), heterozygous (HET), β2 knockout (KO) and tr(CT) mice indicates partial recovery in the tr(CT) line relative to KO. (b) Immunoprecipitation of α4, β2 and α5 nAChR subunits from thalamic samples of each genotype expressed as femtomoles of immunoprecipitated [3H]-epibatidine-labeled nAChR per milligram of protein. Antibodies raised against the cytoplasmic loop of the rat (cytR), carboxyl terminal region of the rat (coohR) and cytoplasmic loop of the mouse (cytM) α5 nAChR subunit were used. (c) Immunoprecipitation results expressed with β2 subunit levels taken as 100%. (d) Immunoprecipitation results expressed with β2 subunit levels taken as 100% and with data from the three α5 subunit antibodies combined, which indicates a significantly (***p<0.001) higher proportion of β2* nAChRs in the tr(CT) thalamus also contain the α5 subunit relative to the other genotypes.