Figure 7.
Ndfip1 Interacts with Itch and Promotes Degradation of JunB
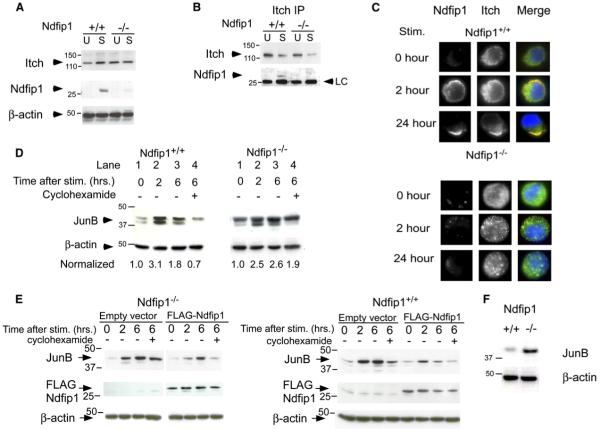
(A) T cells were isolated from the spleens and lymph nodes of Ndfip1+/+ and Ndfip1−/− mice and cultured in media alone or stimulated 24 hr with anti-CD3 and anti-CD28. Cell lysates were immunoblotted with antibodies against Itch, Ndfip1, or β-actin.
(B) Ndfip1+/+ and Ndfip1−/− T cells were lysed directly after isolation (U) or cultured in the presence of anti-CD3 and anti-CD28 (S). Lysates were immunoprecipitated with an anti-Itch antibody and blots were probed for Itch and Ndfip1.
(C) Ndfip1+/+ and Ndfip1−/− T cells were stimulated for the times points indicated and then fixed, permeabilized, and stained for intracellular Itch and Ndfip1.
(D) Ndfip1+/+ and Ndfip1−/− T cells were isolated and depleted for CD44+ cells. Cells were then cultured in media alone or stimulated for 2 or 6 hr or stimulated for 6 hr with cyclohexamide added for the last 4 hr of stimulation. Numbers represent JunB amounts normalized to unstimulated controls for each genotype. Degradation of JunB is measured by comparing lane 4 versus lane 2 for each genotype.
(E) Ndfip1−/− and Ndfip1+/+ T cell lines were transduced with the empty MIG retrovirus (MMLV-IRES-eGFP) or a Flag-tagged Ndfip1 containing MIG retrovirus. GFP+ cells were sorted and JunB degradation was analyzed as described in (D).
(F) T cells were directly isolated from an 8- to 10-week-old Ndfip1+/+ and Ndfip1−/− mice and WCL were analyzed for JunB protein amounts. β-actin is shown as a control for protein loading. LC, light chain.