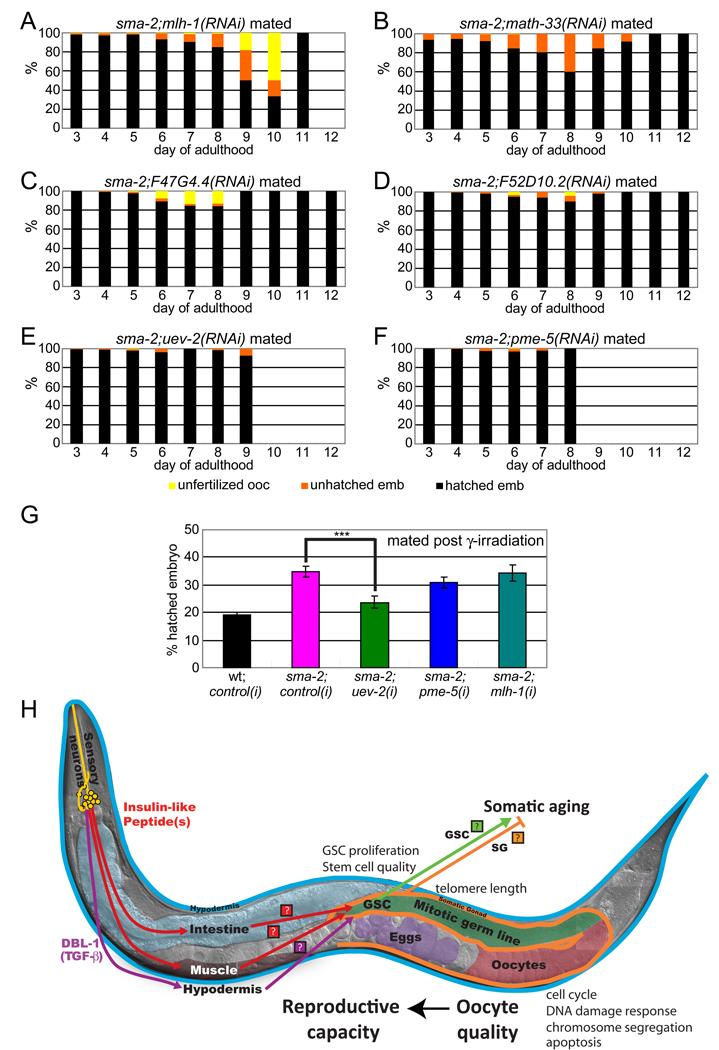
Figure 7. TGF-β Sma/Mab signaling regulates genes important for age-associated oocyte quality maintenance.
(A-F) RNAi treatments of TGF-β target genes accelerate oocyte quality decline, increasing the percentage of unhatched embryos (orange) and/or unfertilized oocytes (yellow) earlier in life (compare with Figure 6C). mlh-1, math-33, and F47G4.4 RNAis have greater effects (A-C), while F52D10.2, uev-2, and pme-5 have milder effects (D-F). Wild type treated with RNAis shown in Figure S7C-F, I-J).
(G) uev-2 RNAi treatment significantly increases sma-2’s production of unhatched embryos after γ-irradiation, while pme-5 and mlh-1 do not. Animals were mated with young wt males after irradiation.
(H) Model of reproductive aging regulation by the TGF-β Sma/Mab (pink) and insulin/IGF-1 signaling (red) pathways. Ligands (Insulin-Like Peptides, TGF-β DBL-1) are secreted neuronally and mediate signaling to the soma (hypodermis, intestine, and muscle), generating as yet unidentified secondary signals to regulate reproduction. These secondary signals block distal germline and oocyte integrity maintenance with age, resulting in germline morphology decline, slowed germ cell proliferation, and a decline in oocyte quality. Downstream effectors in oocytes include chromosome segregation, cell cycle, and DNA damage response/repair genes, etc. Declines in embryonic viability and infertility mark reproductive cessation. The germline and somatic gonad regulate somatic aging (Hsin and Kenyon, 1999), suggesting a bi-directional signaling flow in the coordination of somatic and germline aging. (Photo courtesy of Ian Chin-Sang.)