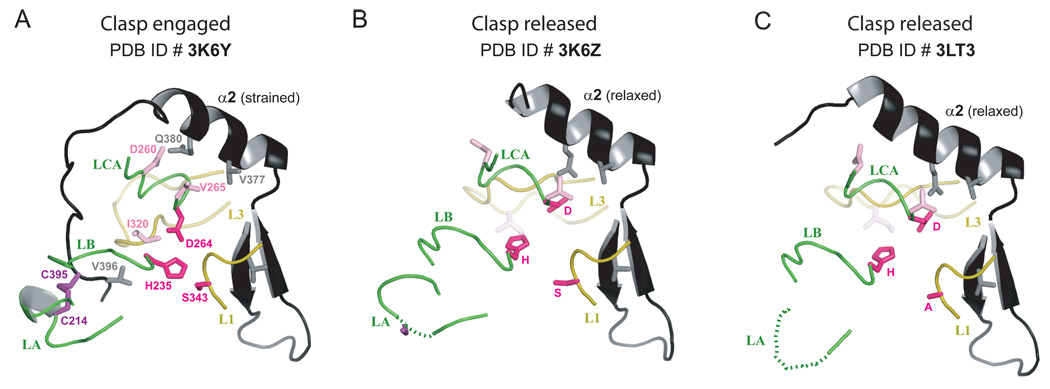
Figure 8.
Cartoon representations of the two conformations of the clasp (in black) in the three Rv3671c structures. Protein loops (loop LA: residues 210–218, loop LB: residues 235–242, loop LCA: residues 258–265, loop L3: residues 316–328 and loop L1: residues 342–346) and the catalytic triad residues are highlighted, demonstrating their concerted conformational changes. A. The active (strained) state of the protease with the clasp engaged, as observed in the structure of Rv3671c_161-397. B,C. The inactive (relaxed) state of the protease with the clasp released, as observed in the structures of Rv3671c_179-397 (panel B) and its Ser343Ala mutant (panel C).