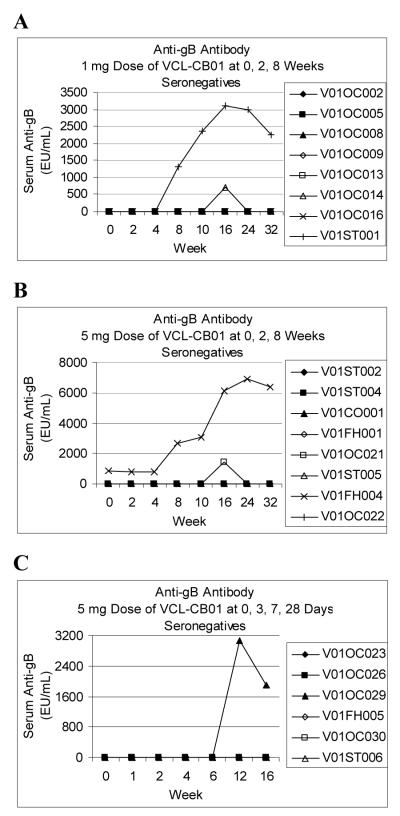
Figure 2. Antibody Responses for CMV-Seronegative Subjects.
Cytomegalovirus (CMV) glycoprotein B (gB) ELISAs were performed as described in Materials and Methods. A serum specimen was positive in the assay if the absorbance for serum at a 1:100 dilution was greater than a reactivity threshold calculated as 2.5 times the absorbance of pooled CMV-seronegative sera at a 1:100 dilution. Anti-gB levels in EU/mL were interpolated from a standard curve using serum with well defined reactivity to CMV gB. In a survey of 76 CMV-seronegative sera and 55 CMV-seropositive sera the assay had a sensitivity of 100% and specificity of 95%. The results for the CMV-seropositive sera ranged from 16,350 EU/mL to 413,440 EU/mL with a median value of 154,560 EU/mL (authors’ unpublished data).
Individual gB antibody responses are shown for CMV-seronegative subjects vaccinated with 1 mg (A), or 5 mg (B) doses of VCL-CB01 at 0, 2, and 8 weeks and CMV-seronegative subjects vaccinated with 5 mg doses of VCL-CB01 at 0, 3, 7, and 28 days (C).