Table 3.
Representative chemical structures and inhibitory activity of the PKCθ inhibitor dataset.
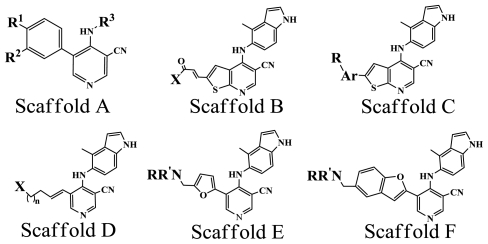 | ||||||
|---|---|---|---|---|---|---|
| No. | Scaffold | Substituent | pIC50 | Refa | ||
| R1 | R2 | R3 | ||||
| 1* | A | OMe | OMe | 3-Bromophenyl | 5.337 | [8] |
| 2 | A | OMe | OMe | Phenyl | 5.796 | [8] |
| 3* | A | OMe | OMe | 3-Chlorophenyl | 5.409 | [8] |
| X | ||||||
| 17 | B | Pyrrolidine | 8.420 | [9] | ||
| 23 | B | H2N | 7.921 | [9] | ||
| 27 | B | PhNH | 6.959 | [9] | ||
| Ar | R | |||||
| 77 | C | Phenyl | 4-CH2-NMe2 | 7.854 | [11] | |
| 80 | C | 3-Pyridine | 5-CH2-NMe2 | 7.076 | [11] | |
| 85* | C | Phenyl | 2-OMe,3-CH2-NMe2 | 7.921 | [11] | |
| X | n | |||||
| 37 | D | 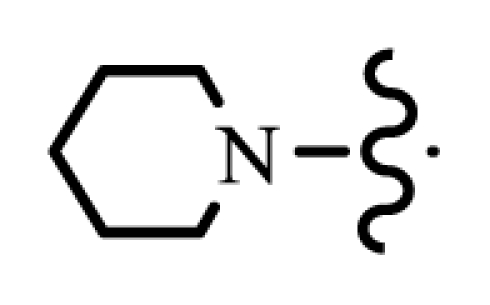 |
1 | 7.456 | [10] | |
| 41* | D | 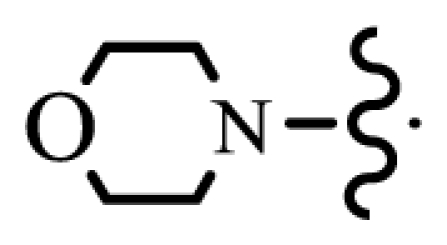 |
2 | 7.469 | [10] | |
| NR’R | ||||||
| 137 | E | Morpholine | 8.108 | [14] | ||
| 140 | E | Pyrrolidine | 7.456 | [14] | ||
| NR’R | ||||||
| 153* | F | Morpholine | 7.886 | [15] | ||
| 157* | F | NHCH2CH(OH)CH2OH | 8.824 | [15] | ||
Test set;
from the corresponding reference.
