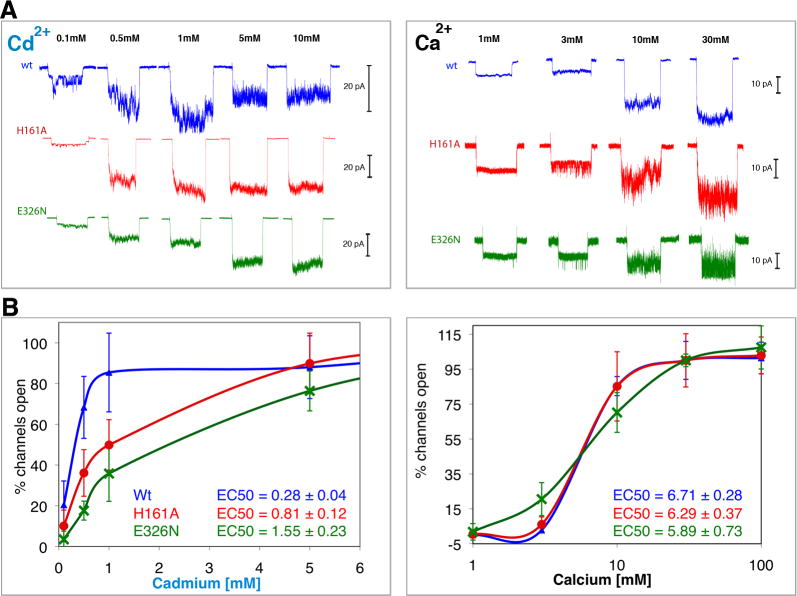
Figure 3.
Involvement of the unique Cd2+ sites in activation of MthK. Potassium currents were measured via patch-clamp experiments on giant E. coli spheroplasts expressing MthK channels activated by Cd2+ (left) and Ca2+ (right). To avoid N-terminal desensitization, Δ2-17Mthk channels were used (A) Typical macroscopic currents observed upon ligand activation of excised patches of wt (blue) and of H161A (red) and E326N (green), the putative knock-outs of the unique Cd2+-bindig Sites 3 and 4, respectively. Each trace corresponds to a single patch activated for 10 seconds, followed by a resting time of at least another 10 seconds before changing the ligand concentration. The maximum number of open channels was reached at ∼1 mM Cd2+, above which inhibition is observed. However, to achieve that with Ca2+ more than 30 mM was required. To better appreciate the effect of the treatments it is necessary to account for the various numbers of channels that exist in different patches. This number was derived dividing the peak amplitude of a patch by the unitary conductance (of a single channel) measured at each ligand concentration as described (Kuo et al., 2007b) (B) The percentages of open channels, averaged from 5 to 10 patches at each concentration (SD are shown as error bars), are plotted against concentrations of Cd2+ and Ca2+ (in logarithmic scale for clarity). These plots show that mutations at H161 and E326 significantly reduce efficiency of activation by Cd2+ but not by Ca2+.