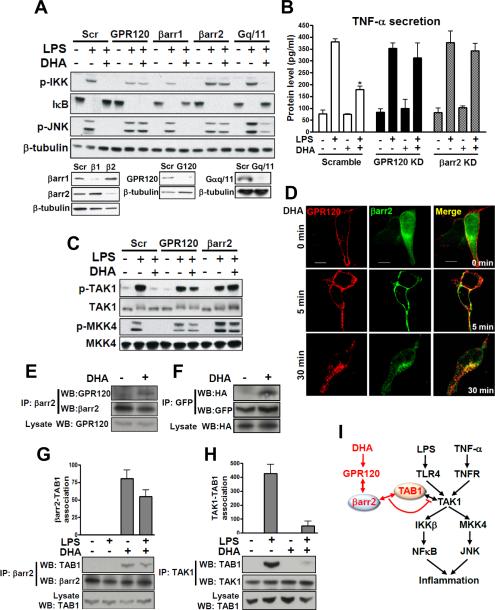
FIG. 3. GPR120 internalization with β-arrestin2 mediates anti-inflammatory effects.
(A) RAW 264.7 cells were transfected with siRNA as indicated and stimulated with or without 100 μM of DHA 1 hr prior to LPS (100 ng/ml) treatment for 10 min and then subjected to western blotting. (B) TNF-α secretion was measured in RAW 264.7 cell cultured media with or without RNA interference as indicated. (C) Phosphorylation of TAK1 and MKK4 in RAW 264.7 cells with or without siRNA transfection as indicated. (D) HEK 293 cells were co-transfected with HA-GPR120 and β-arrestin2·GFP to analyze GPR120 internalization after DHA stimulation for the indicated times. GPR120 (red) and β-arrestin2 (green) were localized by confocal microscopy. (E) Co-immunoprecipitation between GPR120 and β-arrestin2 with DHA stimulation for 30 min in RAW 264.7 cells and, (F) HEK 293 cells (HA-GPR120 and β-arrestin2·GFP), respectively. Lysate indicates 1/10 input in each experiment. Interaction between TAB1 and β-arrestin2 (G), and interaction between TAB1 and TAK1 (H) were detected by co-immunoprecipitation and the scanned bar graph quantitates the association in RAW 264.7 cells. (I) Schematic diagram of the β-arrestin2 and GPR120-mediated anti-inflammatory mechanism. Red colored letters and arrows indicate the DHA-mediated anti-inflammatory effect, and black colored letters and arrows indicate the LPS- and TNF-α-induced inflammatory pathway. See also Fig. S2.