FIGURE 6. Pathologic molar ratios of 4-HNE to unphosphorylated ERK-2 protein result in the accumulation of aldehyde-monomer adducts similar to observations in vivo and in primary culture.
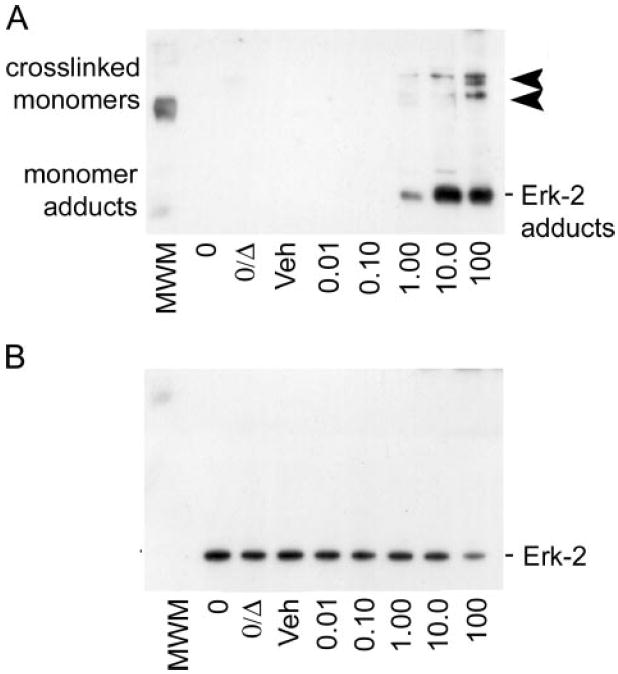
A, immunoblot analysis of inactive ERK-2 protein incubated with increasing physiologic concentrations of 4-HNE and probed for 4-HNE-protein adducts shows the formation of the 4-HNE-ERK-2 monomer adducts as the predominant adducted species (lower, 42-kDa bands). At the highest concentration (100 μm), 4-HNE adduction of unphosphorylated ERK-2 results in chemical cross-linking, as demonstrated by the partial shift from 42 to 82 and 126 kDa (arrowheads) for 4-HNE-adduct-positive staining bands. B, immunoblot analysis of the blot in A, when stripped and reprobed for total ERK-1/2 protein, that shows a partial loss of positive signal that correlates with the cross-linking of ERK-2 monomers at 100 μm 4-HNE. These data suggest that two distinct adduct species exist, where only the 100 μm 4-HNE treatment results in cross-linked adducts that preclude the recognition of the epitope by the total ERK-1/2 antibody.
