Table 1.
Interaction of thiophenols with perfluoroalkyl iodides in liquid ammonia under UV irradiation.
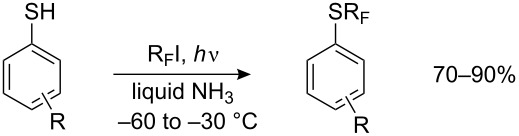 | |||
| R | RF | Yields of ArSRF, % | Ref. |
| H | CF3 | 76 | [143] |
| C2F5, n-C3F7, iso-C3F7 | 84, 81, 76 | [144] | |
| 4-NH2 | CF3 | 87 | [146] |
| 2-NH2 | CF3 | 71 | [143] |
| 4-OH | CF3 | 69.5 | [143] |
| 2-OCH3 | CF3 | 86 | [98] |
| 4-Cl | CF3 | 72 | [146] |
| C2F5, n-C3F7, iso-C3F7 | 84, 83, 65 | [144] | |
| 2-SO2CHF2 | CF3 | 69 | [143,146] |
| 4-SO2CF3 | CF3 | 78 | [143,146] |
| 4-NO2 | CF3 | 2.7a | [143,146] |
| 63b | [143,146] | ||
| 2,4-Cl2 | CF3 | 87 | [149] |
| C3F7 | 89 | [149] | |
| 2-COOH | CF3 | 90 | [150] |
| 3- and 4-COOCH3 | CF3, n-C3F7, iso-C3F7 | 70–80 | [151] |
| 3- and 4-F | CF3, n-C3F7 | 80–90 | [152] |
| iso-C3F7 | 72–75 | [152] | |
| 4-NHCOCH3 | CF3 | 96 | [153] |
| 4-NHCOOCH3 | CF3, n-C3F7 | 88 (92c), 82 (93c) | [9] |
| C2F5, C4F9 | 62, 55 | [154] | |
aIn a quartz flask.
bIn a quartz ampoule at 30–45 °C.
cWith preliminary reduction of 4,4′-bis(MeOCONH)diaryl disulfide and without the isolation of corresponding thiophenol.
