Table 6.
Reactions of thiophenoxides with CF3Br under UV irradiation and pressure of reaction gas [179].
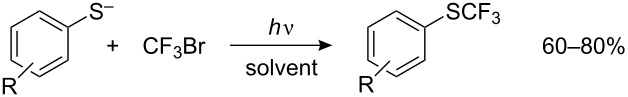 | |||||||
| R | Solvent | Base | p (atm) | T (°C) | Irradiation time, (h) | Conversion of ArSH, (%) | Isolated yields of ArSCF3 (%) |
| 4-CH3 | DMF | Et3N | 4–5 | 10–13 | 1.5 | 82 | |
| 4-NH2 | DMF | Et3N | 4.5–6 | 10–20 | 2 | 76.4 | |
| 3-NH2 | HMPA | morpholine | 3–4 | 17–19 | 3.25 | 63.5a | |
| 4-NHCOMe | DMF | Et3N | 3.5 | 19 | 2.7 | 69 | |
| 4-NHCO2Me | DMF | Et3N | 4.5–5 | 15–25 | 1.2 | 63 | 55.5 |
| HMPA | morpholine | 2–5 | 8–10 | 2.5 | 73 | 83.6 | |
| 4-Cl | CH3CN | Et3N | 3–3.5 | 15–18 | 2.8 | 53 | 43a |
| DMF | Et3N | 3–3.5 | 14 | 1.2 | 100 | 48a | |
| HMPA | Et3N | 4 | 8–10 | 1 | 100 | 69 | |
| HMPA | morpholine | 3–4 | 14–16 | 3.5 | 97 | 62.5 | |
| HMPA | morpholine | 3–4.5 | 29–30 | 3 | 36 | 46 | |
| Sulfolane | morpholine | 3.5 | 23 | 2 | 19.5 | 5.4 | |
| N-Methyl pyrrolidone | morpholine | 3.5 | 17 | 2.2 | 35.5 | 14.3 | |
aDetermined by GLC.
