Table 8.
Preparation of aminophenyl trifluoromethyl sulfides with CF3Br (3–7 atm) and UV irradiation with preliminary reduction of dinitrodiphenyl disulfides [179].
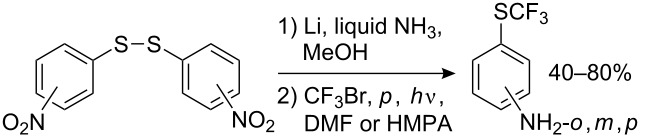 | |||||
| Location of NO2 (NH2) | Solvents | p (atm) | T (°C) | Irradiation time, h | Yields of products, % |
| o- | DMF | 4.6–6 | 10–13 | 7.75 | 40.9 |
| m- | DMF | 3–3.5 | 8–10 | 2.2 | 56a |
| DMF | 3–6 | 10–14 | 4 | 72.5a | |
| DMF | 4–6 | 12–19 | 6.8 | 80.8 | |
| HMPA | 3–5 | 8–10 | 3 | 71.8a | |
| p- | DMF | 5–6 | 15–20 | 5 | 80.3 |
aIsolated as the acetyl derivative.
Due to greater UV stability of CF3Br compared to CF3I, it is possible to increase the irradiation time, with a beneficial effect on the product yield.
